Abstract
Mutations within the gene encoding for the chloride ion channel cystic fibrosis transmembrane conductance regulator (CFTR) results in cystic fibrosis (CF), the most common lethal autosomal recessive genetic disease that causes a number of long-term health problems, as the bone disease. Osteoporosis and increased vertebral fracture risk associated with CF disease are becoming more important as the life expectancy of patients continues to improve. The etiology of low bone density is multifactorial, most probably a combination of inadequate peak bone mass during puberty and increased bone losses in adults. Body mass index, male sex, advanced pulmonary disease, malnutrition and chronic therapies are established additional risk factors for CF-related bone disease (CFBD). Consistently, recent evidence has confirmed that CFTR plays a major role in the osteoprotegerin (OPG) and COX-2 metabolite prostaglandin E2 (PGE2) production, two key regulators in the bone formation and regeneration. Several others mechanisms were also recognized from animal and cell models contributing to malfunctions of osteoblast (cell that form bone) and indirectly of bone-resorpting osteoclasts. Understanding such mechanisms is crucial for the development of therapies in CFBD. Innovative therapeutic approaches using CFTR modulators such as C18 have recently shown in vitro capacity to enhance PGE2 production and normalized the RANKL-to-OPG ratio in human osteoblasts bearing the mutation F508del-CFTR and therefore potential clinical utility in CFBD. This review focuses on the recently identified pathogenic mechanisms leading to CFBD and potential future therapies for treating CFBD.


Similar content being viewed by others
References
Riordan JR, Rommens JM, Kerem B, Alon N, Rozmahel R, Grzelczak Z, Zielenski J, Lok S, Plavsic N, Chou JL et al (1989) Identification of the cystic fibrosis gene: cloning and characterization of complementary DNA [published erratum appears in Science 1989 Sep 29;245(4925):1437]. Science 245:1066–1073
Stoltz DA, Meyerholz DK, Welsh MJ (2015) Origins of cystic fibrosis lung disease. N Engl J Med 372:351–362
Boyle MP, De Boeck K (2013) A new era in the treatment of cystic fibrosis: correction of the underlying CFTR defect. Lancet Respir Med 1:158–163
Riordan JR (2008) CFTR function and prospects for therapy. Annu Rev Biochem 77:701–726
Cheng SH, Gregory RJ, Marshall J, Paul S, Souza DW, White GA, O’Riordan CR, Smith AE (1990) Defective intracellular transport and processing of CFTR is the molecular basis of most cystic fibrosis. Cell 63:827–834
Boucher RC (2004) New concepts of the pathogenesis of cystic fibrosis lung disease. Eur Respir J 23:146–158
O’Sullivan BP, Freedman SD (2009) Cystic fibrosis. Lancet 373:1891–1904
Kelly A, Moran A (2013) Update on cystic fibrosis-related diabetes. J Cyst Fibros 12:318–331
Rowland M, Bourke B (2011) Liver disease in cystic fibrosis. Curr Opin Pulm Med 17:461–466
Plant BJ, Goss CH, Plant WD, Bell SC (2013) Management of comorbidities in older patients with cystic fibrosis. Lancet Respir Med 1:164–174
Stalvey MS, Clines GA (2013) Cystic fibrosis-related bone disease: insights into a growing problem. Curr Opin Endocrinol Diabetes Obes 20:547–552
Conwell LS, Chang AB (2014) Bisphosphonates for osteoporosis in people with cystic fibrosis. Cochrane Database Syst Rev 3:CD002010
Haworth CS, Webb AK, Egan JJ, Selby PL, Hasleton PS, Bishop PW, Freemont TJ (2000) Bone histomorphometry in adult patients with cystic fibrosis. Chest 118:434–439
Javier RM, Jacquot J (2011) Bone disease in cystic fibrosis: what’s new? Joint Bone Spine 78:445–450
Le Heron L, Guillaume C, Velard F, Braux J, Touqui L, Moriceau S, Sermet-Gaudelus I, Laurent-Maquin D, Jacquot J (2010) Cystic fibrosis transmembrane conductance regulator (CFTR) regulates the production of osteoprotegerin (OPG) and prostaglandin (PG) E(2) in human bone. J Cyst Fibros 9:69–72
Shead EF, Haworth CS, Condliffe AM, McKeon DJ, Scott MA, Compston JE (2007) Cystic fibrosis transmembrane conductance regulator (CFTR) is expressed in human bone. Thorax 62:650–651
Aris RM, Merkel PA, Bachrach LK, Borowitz DS, Boyle MP, Elkin SL, Guise TA, Hardin DS, Haworth CS, Holick MF, Joseph PM, O’Brien K, Tullis E, Watts NB, White TB (2005) Guide to bone health and disease in cystic fibrosis. J Clin Endocrinol Metab 90:1888–1896
Bianchi ML, Romano G, Saraifoger S, Costantini D, Limonta C, Colombo C (2006) BMD and body composition in children and young patients affected by cystic fibrosis. J Bone Miner Res 21:388–396
Sermet-Gaudelus I, Souberbielle JC, Ruiz JC, Vrielynck S, Heuillon B, Azhar I, Cazenave A, Lawson-Body E, Chedevergne F, Lenoir G (2007) Low bone mineral density in young children with cystic fibrosis. Am J Respir Crit Care Med 175:951–957
Paccou J, Zeboulon N, Combescure C, Gossec L, Cortet B (2010) The prevalence of osteoporosis, osteopenia, and fractures among adults with cystic fibrosis: a systematic literature review with meta-analysis. Calcif Tissue Int 86:1–7
Ambroszkiewicz J, Sands D, Gajewska J, Chelchowska M, Laskowska-Klita T (2013) Bone turnover markers, osteoprotegerin and RANKL cytokines in children with cystic fibrosis. Adv Med Sci 58:338–343
Sermet-Gaudelus I, Bianchi ML, Garabedian M, Aris RM, Morton A, Hardin DS, Elkin SL, Compston JE, Conway SP, Castanet M, Wolfe S, Haworth CS (2011) European cystic fibrosis bone mineralisation guidelines. J Cyst Fibros 10(Suppl 2):S16–23
Legroux-Gerot I, Leroy S, Prudhomme C, Perez T, Flipo RM, Wallaert B, Cortet B (2011) Bone loss in adults with cystic fibrosis: prevalence, associated factors, and usefulness of biological markers. Joint Bone Spine 79:73–77
Rossini M, Del Marco A, Dal Santo F, Gatti D, Braggion C, James G, Adami S (2004) Prevalence and correlates of vertebral fractures in adults with cystic fibrosis. Bone 35:771–776
Tejero Garcia S, Giraldez Sanchez MA, Cejudo P, Quintana Gallego E, Dapena J, Garcia Jimenez R, Cano Luis P, Gomez de Terreros I (2011) Bone health, daily physical activity, and exercise tolerance in patients with cystic fibrosis. Chest 140:475–481
Hernandez CJ, Keaveny TM (2006) A biomechanical perspective on bone quality. Bone 39:1173–1181
Gore AP, Kwon SH, Stenbit AE (2010) A roadmap to the brittle bones of cystic fibrosis. J Osteoporos 2011:926045
Haworth CS (2010) Impact of cystic fibrosis on bone health. Curr Opin Pulm Med 16:616–622
Elkin SL, Fairney A, Burnett S, Kemp M, Kyd P, Burgess J, Compston JE, Hodson ME (2001) Vertebral deformities and low bone mineral density in adults with cystic fibrosis: a cross-sectional study. Osteoporos Int 12:366–372
Shead EF, Haworth CS, Gunn E, Bilton D, Scott MA, Compston JE (2006) Osteoclastogenesis during infective exacerbations in patients with cystic fibrosis. Am J Respir Crit Care Med 174:306–311
Alicandro G, Bisogno A, Battezzati A, Bianchi ML, Corti F, Colombo C (2014) Recurrent pulmonary exacerbations are associated with low fat free mass and low bone mineral density in young adults with cystic fibrosis. J Cyst Fibros 13:328–334
Velard F, Delion M, Le Henaff C, Guillaume C, Gangloff S, Jacquot J, Tabary O, Touqui L, Barthes F, Sermet-Gaudelus I (2014) Cystic fibrosis and bone disease: defective osteoblast maturation with the F508del mutation in cystic fibrosis transmembrane conductance regulator. Am J Respir Crit Care Med 189:746–748
Boyle MP (2006) Update on maintaining bone health in cystic fibrosis. Curr Opin Pulm Med 12:453–458
King SJ, Topliss DJ, Kotsimbos T, Nyulasi IB, Bailey M, Ebeling PR, Wilson JW (2005) Reduced bone density in cystic fibrosis: DeltaF508 mutation is an independent risk factor. Eur Respir J 25:54–61
Scheid P, Kempster L, Griesenbach U, Davies JC, Dewar A, Weber PP, Colledge WH, Evans MJ, Geddes DM, Alton EW (2001) Inflammation in cystic fibrosis airways: relationship to increased bacterial adherence. EurRespirJ 17:27–35
Elkin SL, Vedi S, Bord S, Garrahan NJ, Hodson ME, Compston JE (2002) Histomorphometric analysis of bone biopsies from the iliac crest of adults with cystic fibrosis. Am J Respir Crit Care Med 166:1470–1474
Putman MS, Milliren CE, Derrico N, Uluer A, Sicilian L, Lapey A, Sawicki G, Gordon CM, Bouxsein ML, Finkelstein JS (2014) Compromised bone microarchitecture and estimated bone strength in young adults with cystic fibrosis. J Clin Endocrinol Metab 99:3399–3407
Le Henaff C, Hay E, Velard F, Marty C, Tabary O, Marie PJ, Jacquot JP (2014) Enhanced F508del-CFTR channel activity ameliorates bone pathology in murine cystic fibrosis. Am J Pathol 184:1132–1141
Stalvey MS, Clines KL, Havasi V, McKibbin CR, Dunn LK, Chung WJ, Clines GA (2013) Osteoblast CFTR inactivation reduces differentiation and osteoprotegerin expression in a mouse model of cystic fibrosis-related bone disease. PLoS One 8:e80098
Bonora M, Riffault L, Marie S, Mall M, Clement A, Tabary O (2011) Morphological analysis of the trachea and pattern of breathing in betaENaC-Tg mice. Respir Physiol Neurobiol 178:346–348
Bonvin E, Le Rouzic P, Bernaudin JF, Cottart CH, Vandebrouck C, Crie A, Leal T, Clement A, Bonora M (2008) Congenital tracheal malformation in cystic fibrosis transmembrane conductance regulator-deficient mice. J Physiol 586:3231–3243
Meyerholz DK, Stoltz DA, Namati E, Ramachandran S, Pezzulo AA, Smith AR, Rector MV, Suter MJ, Kao S, McLennan G, Tearney GJ, Zabner J, McCray PB Jr, Welsh MJ (2010) Loss of cystic fibrosis transmembrane conductance regulator function produces abnormalities in tracheal development in neonatal pigs and young children. Am J Respir Crit Care Med 182:1251–1261
Adam RJ, Michalski AS, Bauer C, Abou Alaiwa MH, Gross TJ, Awadalla MS, Bouzek DC, Gansemer ND, Taft PJ, Hoegger MJ, Diwakar A, Ochs M, Reinhardt JM, Hoffman EA, Beichel RR, Meyerholz DK, Stoltz DA (2013) Air trapping and airflow obstruction in newborn cystic fibrosis piglets. Am J Respir Crit Care Med 188:1434–1441
Davies JC, Wainwright CE, Canny GJ, Chilvers MA, Howenstine MS, Munck A, Mainz JG, Rodriguez S, Li H, Yen K, Ordonez CL, Ahrens R (2013) Efficacy and safety of ivacaftor in patients aged 6 to 11 years with cystic fibrosis with a G551D mutation. Am J Respir Crit Care Med 187:1219–1225
Wainwright CE, Elborn JS, Ramsey BW, Marigowda G, Huang X, Cipolli M, Colombo C, Davies JC, De Boeck K, Flume PA, Konstan MW, McColley SA, McCoy K, McKone EF, Munck A, Ratjen F, Rowe SM, Waltz D, Boyle MP (2015) Lumacaftor-ivacaftor in patients with cystic fibrosis homozygous for Phe508del CFTR. N Engl J Med
Baroncelli GI, De Luca F, Magazzu G, Arrigo T, Sferlazzas C, Catena C, Bertelloni S, Saggese G (1997) Bone demineralization in cystic fibrosis: evidence of imbalance between bone formation and degradation. Pediatr Res 41:397–403
Nicolaidou P, Stavrinadis I, Loukou I, Papadopoulou A, Georgouli H, Douros K, Priftis KN, Gourgiotis D, Matsinos YG, Doudounakis S (2006) The effect of vitamin K supplementation on biochemical markers of bone formation in children and adolescents with cystic fibrosis. Eur J Pediatr 165:540–545
Cohen-Cymberknoh M, Shoseyov D, Kerem E (2011) Managing cystic fibrosis: strategies that increase life expectancy and improve quality of life. Am J Respir Crit Care Med 183:1463–1471
Sermet-Gaudelus I, Castanet M, Retsch-Bogart G, Aris RM (2009) Update on cystic fibrosis-related bone disease: a special focus on children. Paediatr Respir Rev 10:134–142
Putman MS, Baker JF, Uluer A, Herlyn K, Lapey A, Sicilian L, Tillotson AP, Gordon CM, Merkel PA, Finkelstein JS (2015) Trends in bone mineral density in young adults with cystic fibrosis over a 15-year period. J Cyst Fibros
Giustina A, Mazziotti G, Canalis E (2008) Growth hormone, insulin-like growth factors, and the skeleton. Endocr Rev 29:535–559
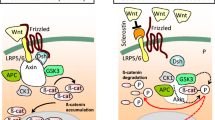
Baron R, Kneissel M (2013) WNT signaling in bone homeostasis and disease: from human mutations to treatments. Nat Med 19:179–192
Festini F, Taccetti G, Repetto T, Reali MF, Campana S, Mergni G, Marianelli L, de Martino M (2005) Gestational and neonatal characteristics of children with cystic fibrosis: a cohort study. J Pediatr 147:316–320
Switzer M, Rice J, Rice M, Hardin DS (2009) Insulin-like growth factor-I levels predict weight, height and protein catabolism in children and adolescents with cystic fibrosis. J Pediatr Endocrinol Metab 22:417–424
Rogan MP, Reznikov LR, Pezzulo AA, Gansemer ND, Samuel M, Prather RS, Zabner J, Fredericks DC, McCray PB Jr, Welsh MJ, Stoltz DA (2010) Pigs and humans with cystic fibrosis have reduced insulin-like growth factor 1 (IGF1) levels at birth. Proc Natl Acad Sci U S A 107:20571–20575
Haworth CS, Freemont AJ, Webb AK, Dodd ME, Selby PL, Mawer EB, Adams JE (1999) Hip fracture and bone histomorphometry in a young adult with cystic fibrosis. Eur Respir J 14:478–479
Imai Y, Kondoh S, Kouzmenko A, Kato S (2011) Minireview: osteoprotective action of estrogens is mediated by osteoclastic estrogen receptor-alpha. Mol Endocrinol 24:877–885
Chen H, Guo JH, Lu YC, Ding GL, Yu MK, Tsang LL, Fok KL, Liu XM, Zhang XH, Chung YW, Huang P, Huang H, Chan HC (2012) Impaired CFTR-dependent amplification of FSH-stimulated estrogen production in cystic fibrosis and PCOS. J Clin Endocrinol Metab 97:923–932
Gimenez A, Le Henaff C, Norez C, Guillaume C, Ravoninjatovo B, Laurent-Maquin D, Becq F, Jacquot J (2012) Deficit of osteoprotegerin release by osteoblasts from a patient with cystic fibrosis. Eur Respir J 39:780–781
Simonet WS, Lacey DL, Dunstan CR, Kelley M, Chang MS, Luthy R, Nguyen HQ, Wooden S, Bennett L, Boone T, Shimamoto G, DeRose M, Elliott R, Colombero A, Tan HL, Trail G, Sullivan J, Davy E, Bucay N, Renshaw-Gegg L, Hughes TM, Hill D, Pattison W, Campbell P, Sander S, Van G, Tarpley J, Derby P, Lee R, Boyle WJ (1997) Osteoprotegerin: a novel secreted protein involved in the regulation of bone density. Cell 89:309–319
Pashuck TD, Franz SE, Altman MK, Wasserfall CH, Atkinson MA, Wronski TJ, Flotte TR, Stalvey MS (2009) Murine model for cystic fibrosis bone disease demonstrates osteopenia and sex-related differences in bone formation. Pediatr Res 65:311–316
Snouwaert JN, Brigman KK, Latour AM, Malouf NN, Boucher RC, Smithies O, Koller BH (1992) An animal model for cystic fibrosis made by gene targeting. Science 257:1083–1088
Dif F, Marty C, Baudoin C, de Vernejoul MC, Levi G (2004) Severe osteopenia in CFTR-null mice. Bone 35:595–603
Haston CK, Li W, Li A, Lafleur M, Henderson JE (2008) Persistent osteopenia in adult cystic fibrosis transmembrane conductance regulator-deficient mice. Am J Respir Crit Care Med 177:309–315
Le Henaff C, Gimenez A, Hay E, Marty C, Marie P, Jacquot J (2012) The F508del mutation in cystic fibrosis transmembrane conductance regulator gene impacts bone formation. Am J Pathol 180:2068–2075
Pastores GM, Elstein D, Hrebicek M, Zimran A (2007) Effect of miglustat on bone disease in adults with type 1 Gaucher disease: a pooled analysis of three multinational, open-label studies. Clin Ther 29:1645–1654
Venier RE, Igdoura SA (2012) Miglustat as a therapeutic agent: prospects and caveats. J Med Genet 49:591–597
Galanaud D, Tourbah A, Lehericy S, Leveque N, Heron B, Billette de Villemeur T, Guffon N, Feillet F, Baumann N, Vanier MT, Sedel F (2009) 24 month-treatment with miglustat of three patients with Niemann-Pick disease type C: follow up using brain spectroscopy. Mol Genet Metab 96:55–58
Goessling W, North TE, Loewer S, Lord AM, Lee S, Stoick-Cooper CL, Weidinger G, Puder M, Daley GQ, Moon RT, Zon LI (2009) Genetic interaction of PGE2 and Wnt signaling regulates developmental specification of stem cells and regeneration. Cell 136:1136–1147
Napimoga MH, Demasi AP, Bossonaro JP, de Araujo VC, Clemente-Napimoga JT, Martinez EF (2013) Low doses of 15d-PGJ2 induce osteoblast activity in a PPAR-gamma independent manner. Int Immunopharmacol 16:131–138
Blackwell KA, Raisz LG, Pilbeam CC (2010) Prostaglandins in bone: bad cop, good cop? Trends Endocrinol Metab 21:294–301
Xie C, Liang B, Xue M, Lin AS, Loiselle A, Schwarz EM, Guldberg RE, O’Keefe RJ, Zhang X (2009) Rescue of impaired fracture healing in COX-2−/− mice via activation of prostaglandin E2 receptor subtype 4. Am J Pathol 175:772–785
Baudouin-Legros M, Colas J, Moriceau S, Kelly M, Planelles G, Edelman A, Ollero M (2012) Long-term CFTR inhibition modulates 15d-prostaglandin J2 in human pulmonary cells. Int J Biochem Cell Biol 44:1009–1018
Walker NM, Badri LN, Wadhwa A, Wettlaufer S, Peters-Golden M, Lama VN (2012) Prostaglandin E2 as an inhibitory modulator of fibrogenesis in human lung allografts. Am J Respir Crit Care Med 185:77–84
Nemeth K, Leelahavanichkul A, Yuen PS, Mayer B, Parmelee A, Doi K, Robey PG, Leelahavanichkul K, Koller BH, Brown JM, Hu X, Jelinek I, Star RA, Mezey E (2009) Bone marrow stromal cells attenuate sepsis via prostaglandin E(2)-dependent reprogramming of host macrophages to increase their interleukin-10 production. Nat Med 15:42–49
Diamond JM, Akimova T, Kazi A, Shah RJ, Cantu E, Feng R, Levine MH, Kawut SM, Meyer NJ, Lee JC, Hancock WW, Aplenc R, Ware LB, Palmer SM, Bhorade S, Lama VN, Weinacker A, Orens J, Wille K, Crespo M, Lederer DJ, Arcasoy S, Demissie E, Christie JD (2014) Genetic variation in the prostaglandin E2 pathway is associated with primary graft dysfunction. Am J Respir Crit Care Med 189:567–575
Maier NK, Leppla SH, Moayeri M (2015) The cyclopentenone prostaglandin 15d-PGJ2 inhibits the NLRP1 and NLRP3 inflammasomes. J Immunol 194:2776–2785
Straus DS, Pascual G, Li M, Welch JS, Ricote M, Hsiang CH, Sengchanthalangsy LL, Ghosh G, Glass CK (2000) 15-deoxy-delta 12,14-prostaglandin J2 inhibits multiple steps in the NF-kappa B signaling pathway. Proc Natl Acad Sci U S A 97:4844–4849
Surh YJ, Na HK, Park JM, Lee HN, Kim W, Yoon IS, Kim DD (2011) 15-Deoxy-delta(1)(2), (1)(4)-prostaglandin J(2), an electrophilic lipid mediator of anti-inflammatory and pro-resolving signaling. Biochem Pharmacol 82:1335–1351
Kim KR, Kim HJ, Lee SK, Ma GT, Park KK, Chung WY (2015) 15-Deoxy-delta12,14-prostaglandin j2 inhibits osteolytic breast cancer bone metastasis and estrogen deficiency-induced bone loss. PLoS One 10:e0122764
During A, Penel G, Hardouin P (2015) Understanding the local actions of lipids in bone physiology. Prog Lipid Res
Teichgraber V, Ulrich M, Endlich N, Riethmuller J, Wilker B, De Oliveira-Munding CC, van Heeckeren AM, Barr ML, von Kurthy G, Schmid KW, Weller M, Tummler B, Lang F, Grassme H, Doring G, Gulbins E (2008) Ceramide accumulation mediates inflammation, cell death and infection susceptibility in cystic fibrosis. Nat Med 14:382–391
Strandvik B (2010) Fatty acid metabolism in cystic fibrosis. Prostaglandins Leukot Essent Fatty Acids 83:121–129
Worgall TS, Veerappan A, Sung B, Kim BI, Weiner E, Bholah R, Silver RB, Jiang XC, Worgall S (2011) Impaired sphingolipid synthesis in the respiratory tract induces airway hyperreactivity. Sci Transl Med 5:186ra167
Xu Y, Krause A, Limberis M, Worgall TS, Worgall S (2011) Low sphingosine-1-phosphate impairs lung dendritic cells in cystic fibrosis. Am J Respir Cell Mol Biol 48:250–257
Spiegel S, Milstien S (2003) Sphingosine-1-phosphate: an enigmatic signalling lipid. Nat Rev Mol Cell Biol 4:397–407
Petrie Aronin CE, Shin SJ, Naden KB, Rios PD Jr, Sefcik LS, Zawodny SR, Bagayoko ND, Cui Q, Khan Y, Botchwey EA (2010) The enhancement of bone allograft incorporation by the local delivery of the sphingosine 1-phosphate receptor targeted drug FTY720. Biomaterials 31:6417–6424
Ahn SH, Koh JM, Gong EJ, Byun S, Lee SY, Kim BJ, Lee SH, Chang JS, Kim GS (2013) Association of bone marrow sphingosine 1-phosphate levels with osteoporotic hip fractures. J Bone Metab 20:61–65
Kim BJ, Koh JM, Lee SY, Lee YS, Lee SH, Lim KH, Cho EH, Kim SW, Kim TH, Kim SY, Kim GS (2012) Plasma sphingosine 1-phosphate levels and the risk of vertebral fracture in postmenopausal women. J Clin Endocrinol Metab 97:3807–3814
Matsuzaki E, Hiratsuka S, Hamachi T, Takahashi-Yanaga F, Hashimoto Y, Higashi K, Kobayashi M, Hirofuji T, Hirata M, Maeda K (2013) Sphingosine-1-phosphate promotes the nuclear translocation of beta-catenin and thereby induces osteoprotegerin gene expression in osteoblast-like cell lines. Bone 55:315–324
Malik FA, Meissner A, Semenkov I, Molinski S, Pasyk S, Ahmadi S, Bui HH, Bear CE, Lidington D, Bolz SS (2015) Sphingosine-1-phosphate is a novel regulator of cystic fibrosis transmembrane conductance regulator (CFTR) activity. PLoS One 10:e0130313
Meissner A, Yang J, Kroetsch JT, Sauve M, Dax H, Momen A, Noyan-Ashraf MH, Heximer S, Husain M, Lidington D, Bolz SS (2012) Tumor necrosis factor-alpha-mediated downregulation of the cystic fibrosis transmembrane conductance regulator drives pathological sphingosine-1-phosphate signaling in a mouse model of heart failure. Circulation 125:2739–2750
Lotinun S, Kiviranta R, Matsubara T, Alzate JA, Neff L, Luth A, Koskivirta I, Kleuser B, Vacher J, Vuorio E, Horne WC, Baron R (2013) Osteoclast-specific cathepsin K deletion stimulates S1P-dependent bone formation. J Clin Invest 123:666–681
Zhang JN, Zhao Y, Liu C, Han ES, Yu X, Lidington D, Bolz SS, You L (2015) The role of the sphingosine-1-phosphate signaling pathway in osteocyte mechanotransduction. Bone 79:71–78
Ferron M, Lacombe J (2014) Regulation of energy metabolism by the skeleton: osteocalcin and beyond. Arch Biochem Biophys 561:137–146
Karsenty G, Oury F (2013) Regulation of male fertility by the bone-derived hormone osteocalcin. Mol Cell Endocrinol 382:521–526
Oury F, Ferron M, Huizhen W, Confavreux C, Xu L, Lacombe J, Srinivas P, Chamouni A, Lugani F, Lejeune H, Kumar TR, Plotton I, Karsenty G (2013) Osteocalcin regulates murine and human fertility through a pancreas-bone-testis axis. J Clin Invest 123:2421–2433
Karsenty G, Ferron M (2012) The contribution of bone to whole-organism physiology. Nature 481:314–320
Lee NJ, Nguyen AD, Enriquez RF, Luzuriaga J, Bensellam M, Laybutt R, Baldock PA, Herzog H (2015) NPY signalling in early osteoblasts controls glucose homeostasis. Mol Metab 4:164–174
Ferron M, Hinoi E, Karsenty G, Ducy P (2008) Osteocalcin differentially regulates beta cell and adipocyte gene expression and affects the development of metabolic diseases in wild-type mice. Proc Natl Acad Sci U S A 105:5266–5270
Lee NK, Sowa H, Hinoi E, Ferron M, Ahn JD, Confavreux C, Dacquin R, Mee PJ, McKee MD, Jung DY, Zhang Z, Kim JK, Mauvais-Jarvis F, Ducy P, Karsenty G (2007) Endocrine regulation of energy metabolism by the skeleton. Cell 130:456–469
Rodriguez-Carballo E, Gamez B, Mendez-Lucas A, Sanchez-Freutrie M, Zorzano A, Bartrons R, Alcantara S, Perales JC, Ventura F (2015) p38alpha function in osteoblasts influences adipose tissue homeostasis. Faseb J 29:1414–1425
Wei J, Ferron M, Clarke CJ, Hannun YA, Jiang H, Blaner WS, Karsenty G (2014) Bone-specific insulin resistance disrupts whole-body glucose homeostasis via decreased osteocalcin activation. J Clin Invest 124:1–13
Price PA, Otsuka AA, Poser JW, Kristaponis J, Raman N (1976) Characterization of a gamma-carboxyglutamic acid-containing protein from bone. Proc Natl Acad Sci U S A 73:1447–1451
Lombardi G, Perego S, Luzi L, Banfi G (2014) A four-season molecule: osteocalcin. Updates in its physiological roles. Endocrine 48:394–404
Wei J, Karsenty G (2015) An overview of the metabolic functions of osteocalcin. Curr Osteoporos Rep 13:180–185
Confavreux CB, Borel O, Lee F, Vaz G, Guyard M, Fadat C, Carlier MC, Chapurlat R, Karsenty G (2012) Osteoid osteoma is an osteocalcinoma affecting glucose metabolism. Osteoporos Int 23:1645–1650
Yeap BB, Alfonso H, Chubb SA, Gauci R, Byrnes E, Beilby JP, Ebeling PR, Handelsman DJ, Allan CA, Grossmann M, Norman PE, Flicker L (2015) Higher serum undercarboxylated osteocalcin and other bone turnover markers are associated with reduced diabetes risk and lower estradiol concentrations in older men. J Clin Endocrinol Metab 100:63–71
Rana M, Munns CF, Selvadurai H, Briody J, Craig ME (2013) The impact of dysglycaemia on bone mineral accrual in young people with cystic fibrosis. Clin Endocrinol (Oxf) 78:36–42
Gounarides JS, Korach-Andre M, Killary K, Argentieri G, Turner O, Laurent D (2008) Effect of dexamethasone on glucose tolerance and fat metabolism in a diet-induced obesity mouse model. Endocrinology 149:758–766
Weinstein RS (2011) Clinical practice. Glucocorticoid-induced bone disease. N Engl J Med 365:62–70
Prummel MF, Wiersinga WM, Lips P, Sanders GT, Sauerwein HP (1991) The course of biochemical parameters of bone turnover during treatment with corticosteroids. J Clin Endocrinol Metab 72:382–386
Brennan-Speranza TC, Henneicke H, Gasparini SJ, Blankenstein KI, Heinevetter U, Cogger VC, Svistounov D, Zhang Y, Cooney GJ, Buttgereit F, Dunstan CR, Gundberg C, Zhou H, Seibel MJ (2012) Osteoblasts mediate the adverse effects of glucocorticoids on fuel metabolism. J Clin Invest 122:4172–4189
Clowes JA, Riggs BL, Khosla S (2005) The role of the immune system in the pathophysiology of osteoporosis. Immunol Rev 208:207–227
Hamilton EJ, Rakic V, Davis WA, Chubb SA, Kamber N, Prince RL, Davis TM (2009) Prevalence and predictors of osteopenia and osteoporosis in adults with type 1 diabetes. Diabet Med 26:45–52
Ziai S, Coriati A, Gauthier MS, Rabasa-Lhoret R, Richter MV (2014) Could T cells be involved in lung deterioration and hyperglycemia in cystic fibrosis? Diabetes Res Clin Pract 105:22–29
Mueller C, Braag SA, Keeler A, Hodges C, Drumm M, Flotte TR (2011) Lack of cystic fibrosis transmembrane conductance regulator in CD3+ lymphocytes leads to aberrant cytokine secretion and hyperinflammatory adaptive immune responses. Am J Respir Cell Mol Biol 44:922–929
Bruscia EM, Zhang PX, Satoh A, Caputo C, Medzhitov R, Shenoy A, Egan ME, Krause DS (2009) Abnormal trafficking and degradation of TLR4 underlie the elevated inflammatory response in cystic fibrosis. J Immunol 186:6990–6998
Xu Y, Tertilt C, Krause A, Quadri LE, Crystal RG, Worgall S (2009) Influence of the cystic fibrosis transmembrane conductance regulator on expression of lipid metabolism-related genes in dendritic cells. Respir Res 10:26
Tesmer LA, Lundy SK, Sarkar S, Fox DA (2008) Th17 cells in human disease. Immunol Rev 223:87–113
Tan HL, Regamey N, Brown S, Bush A, Lloyd CM, Davies JC (2011) The Th17 pathway in cystic fibrosis lung disease. Am J Respir Crit Care Med 184:252–258
Tiringer K, Treis A, Fucik P, Gona M, Gruber S, Renner S, Dehlink E, Nachbaur E, Horak F, Jaksch P, Doring G, Crameri R, Jung A, Rochat MK, Hormann M, Spittler A, Klepetko W, Akdis CA, Szepfalusi Z, Frischer T, Eiwegger T (2013) A Th17- and Th2-skewed cytokine profile in cystic fibrosis lungs represents a potential risk factor for Pseudomonas aeruginosa infection. Am J Respir Crit Care Med 187:621–629
Yuan FL, Li X, Lu WG, Zhao YQ, Li CW, Li JP, Sun JM, Xu RS (2012) Type 17 T-helper cells might be a promising therapeutic target for osteoporosis. Mol Biol Rep 39:771–774
Tyagi AM, Srivastava K, Mansoori MN, Trivedi R, Chattopadhyay N, Singh D (2012) Estrogen deficiency induces the differentiation of IL-17 secreting Th17 cells: a new candidate in the pathogenesis of osteoporosis. PLoS One 7:e44552
Haworth CS, Selby PL, Webb AK, Martin L, Elborn JS, Sharples LD, Adams JE (2004) Inflammatory related changes in bone mineral content in adults with cystic fibrosis. Thorax 59:613–617
Shead EF, Haworth CS, Barker H, Bilton D, Compston JE (2010) Osteoclast function, bone turnover and inflammatory cytokines during infective exacerbations of cystic fibrosis. J Cyst Fibros 9:93–98
Shmarina G, Pukhalsky A, Petrova N, Zakharova E, Avakian L, Kapranov N, Alioshkin V (2013) TNF gene polymorphisms in cystic fibrosis patients: contribution to the disease progression. J Transl Med 11:19
Li Y, Li A, Strait K, Zhang H, Nanes MS, Weitzmann MN (2007) Endogenous TNFalpha lowers maximum peak bone mass and inhibits osteoblastic Smad activation through NF-kappaB. J Bone Miner Res 22:646–655
Ferguson JH, Chang AB (2012) Vitamin D supplementation for cystic fibrosis. Cochrane Database Syst Rev 4:CD007298
Bianchi ML, Colombo C, Assael BM, Dubini A, Lombardo M, Quattrucci S, Bella S, Collura M, Messore B, Raia V, Poli F, Bini R, Albanese CV, De Rose V, Costantini D, Romano G, Pustorino E, Magazzu G, Bertasi S, Lucidi V, Traverso G, Coruzzo A, Grzejdziak AD (2013) Treatment of low bone density in young people with cystic fibrosis: a multicentre, prospective, open-label observational study of calcium and calcifediol followed by a randomised placebo-controlled trial of alendronate. Lancet Respir Med 1:377–385
Hirao M, Hashimoto J, Ando W, Ono T, Yoshikawa H (2008) Response of serum carboxylated and undercarboxylated osteocalcin to alendronate monotherapy and combined therapy with vitamin K2 in postmenopausal women. J Bone Miner Metab 26:260–264
Neer RM, Arnaud CD, Zanchetta JR, Prince R, Gaich GA, Reginster JY, Hodsman AB, Eriksen EF, Ish-Shalom S, Genant HK, Wang O, Mitlak BH (2001) Effect of parathyroid hormone (1–34) on fractures and bone mineral density in postmenopausal women with osteoporosis. N Engl J Med 344:1434–1441
Body JJ, Gaich GA, Scheele WH, Kulkarni PM, Miller PD, Peretz A, Dore RK, Correa-Rotter R, Papaioannou A, Cumming DC, Hodsman AB (2002) A randomized double-blind trial to compare the efficacy of teriparatide [recombinant human parathyroid hormone (1–34)] with alendronate in postmenopausal women with osteoporosis. J Clin Endocrinol Metab 87:4528–4535
Siwamogsatham O, Stephens K, Tangpricha V (2014) Evaluation of teriparatide for treatment of osteoporosis in four patients with cystic fibrosis: a case series. Case Rep Endocrinol 2014:893589
Ikpa PT, Bijvelds MJ, de Jonge HR (2014) Cystic fibrosis: toward personalized therapies. Int J Biochem Cell Biol 52:192–200
Bell SC, De Boeck K, Amaral MD (2014) New pharmacological approaches for cystic fibrosis: promises, progress, pitfalls. Pharmacol Ther 145:19–34
Clancy JP, Jain M (2012) Personalized medicine in cystic fibrosis: dawning of a new era. Am J Respir Crit Care Med 186:593–597
Birault V, Solari R, Hanrahan J, Thomas DY (2013) Correctors of the basic trafficking defect of the mutant F508del-CFTR that causes cystic fibrosis. Curr Opin Chem Biol 17:353–360
Van Goor F, Hadida S, Grootenhuis PD, Burton B, Stack JH, Straley KS, Decker CJ, Miller M, McCartney J, Olson ER, Wine JJ, Frizzell RA, Ashlock M, Negulescu PA (2011) Correction of the F508del-CFTR protein processing defect in vitro by the investigational drug VX-809. Proc Natl Acad Sci U S A 108:18843–18848
Molinski S, Eckford PD, Pasyk S, Ahmadi S, Chin S, Bear CE (2012) Functional rescue of F508del-CFTR using small molecule correctors. Front Pharmacol 3:160
Eckford PD, Ramjeesingh M, Molinski S, Pasyk S, Dekkers JF, Li C, Ahmadi S, Ip W, Chung TE, Du K, Yeger H, Beekman J, Gonska T, Bear CE (2014) VX-809 and related corrector compounds exhibit secondary activity stabilizing active F508del-CFTR after its partial rescue to the cell surface. Chem Biol
He L, Kota P, Aleksandrov AA, Cui L, Jensen T, Dokholyan NV, Riordan JR (2013) Correctors of DeltaF508 CFTR restore global conformational maturation without thermally stabilizing the mutant protein. Faseb J 27:536–545
Velard F, Delion M, Lemaire F, Tabary O, Guillaume C, Le Pimpec Barthes F, Touqui L, Gangloff S, Sermet-Gaudelus I, Jacquot J (2015) Cystic fibrosis bone disease: is the CFTR corrector C18 an option for therapy? Eur Respir J 45:845–848
Ramsey BW, Davies J, McElvaney NG, Tullis E, Bell SC, Drevinek P, Griese M, McKone EF, Wainwright CE, Konstan MW, Moss R, Ratjen F, Sermet-Gaudelus I, Rowe SM, Dong Q, Rodriguez S, Yen K, Ordonez C, Elborn JS (2011) A CFTR potentiator in patients with cystic fibrosis and the G551D mutation. N Engl J Med 365:1663–1672
Conflict of interests
None.
Support statement
This study was supported in part by funds from the French Cystic Fibrosis Association Vaincre la Mucoviscidose (RF20150501235 to J. Jacquot). M. Delion is a PhD scholarship recipient from the Ministère de l’Enseignement Supérieur et de la Recherche, Paris, France
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Jacquot, J., Delion, M., Gangloff, S. et al. Bone disease in cystic fibrosis: new pathogenic insights opening novel therapies. Osteoporos Int 27, 1401–1412 (2016). https://doi.org/10.1007/s00198-015-3343-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00198-015-3343-3