Abstract
Introduction and hypothesis
We investigated the effects of locally administered human multilineage-differentiating stress enduring (Muse) cells, nontumorigenic pluripotent-like endogenous stem cells, on bladder tissues, function, and nociceptive behavior in a chemically induced Hunner-type interstitial cystitis (HIC)-like rat model without immunosuppressant.
Methods
Chemical cystitis was induced by intravesical instillation of 0.2 N hydrochloride (HCl) for 15 min in female F344 rats. SSEA-3+ Muse cells, SSEA-3− non-Muse cells or Hanks' balanced salt solution (HBSS; vehicle) were injected into the anterior and posterior bladder wall at each 1×104 cells/10 μl 6 h after HCl application. The sham group received HBSS without HCl instillation. Urinary frequency was assessed using metabolic cages, cystometrograms, nociceptive behavior, and histological analysis of the bladder and L6 spinal cord.
Results
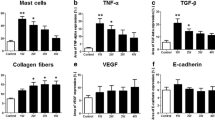
Increases in urinary frequency and decreases in bladder capacity compared with the sham group were observed in the vehicle and non-Muse groups, but not in the Muse group, at 1 week. Significant increases in nociceptive behavior compared with the sham group and the expression of TNFα in the bladder and c-Fos in the bilateral dorsal horns of L6 spinal cord were also observed in the vehicle and non-Muse groups, whereas these changes were not seen in the Muse group at 1 week. Histological analysis exhibited a higher proportion of injected Muse cells remaining in the urothelial basal layer and lamina propria of the bladder than non-Muse cells until 4 weeks.
Conclusions
Muse cell therapy could be a promising modality for treating HIC.




Similar content being viewed by others
Abbreviations
- BC:
-
Bladder capacity
- BM:
-
Bone marrow
- BP:
-
Baseline pressure
- HBSS:
-
Hanks' balanced salt solution
- HCl:
-
Hydrochloride
- HE:
-
Hematoxylin eosin
- HGF:
-
Hepatocyte growth factor
- HIC:
-
Hunner-type interstitial cystitis
- HLA:
-
Human leucocyte antigen
- IC/BPS:
-
Interstitial cystitis/bladder pain syndrome
- MSCs:
-
Mesenchymal stem cells
- Muse:
-
Multilineage-differentiating stress enduring
- MVP:
-
Maximal voiding pressure
- PBS:
-
Phosphate buffered saline
- PE:
-
Polyethylene
- PVR:
-
Post-void residual urine volume
- SD:
-
Standard deviation
- S1PR2:
-
Sphingosine-1-phosphate receptor 2
- SSEA-3:
-
Stage-specific embryonic antigen-3
- TNFα:
-
Tissue necrotic factor α
- TP:
-
Threshold pressure
- UCB:
-
Umbilical cord blood
- VEGF:
-
Vascular endothelial growth factor
References
Homma Y, Akiyama Y, Tomoe H, Furuta A, Ueda T, Maeda D, et al. Clinical guidelines for interstitial cystitis/bladder pain syndrome. Int J Urol. 2020;27:578–89. https://doi.org/10.1111/iju.14234.
Maeda D, Akiyama Y, Morikawa T, Kunita A, Ota Y, Katoh H, et al. Hunner-type (classic) interstitial cystitis: a distinct inflammatory disorder characterized by pancystitis, with frequent expansion of clonal B-cells and epithelial denudation. PLoS One. 2015;10:e0143316. https://doi.org/10.1371/journal.pone.0143316.
Furuta A, Suzuki Y, Igarashi T, Koike Y, Kimura T, Egawa S, et al. Angiogenesis in bladder tissues is strongly correlated with urinary frequency and bladder pain in patients with interstitial cystitis/bladder pain syndrome. Int J Urol. 2019;26(Suppl 1):35–40. https://doi.org/10.1111/iju.13972.
Xiao Y, Song YJ, Song B, Huang CB, Ling Q, Yu X. TGF-β/MAPK signaling mediates the effects of bone marrow mesenchymal stem cells on urinary control and interstitial cystitis after urinary bladder transplantation. Am J Transl Res. 2017;9:1193–202.
Song M, Lim J, Yu HY, Park J, Chun JY, Jeong J, et al. Mesenchymal stem cell therapy alleviates interstitial cystitis by activating Wnt signaling pathway. Stem Cells Dev. 2015;24:1648–57. https://doi.org/10.1089/scd.2014.0459.
Kim A, Yu HY, Heo J, Song M, Shin JH, Lim J, et al. Mesenchymal stem cells protect against the tissue fibrosis of ketamine-induced cystitis in rat bladder. Sci Rep. 2016;6:30881. https://doi.org/10.1038/srep30881.
Furuta A, Yamamoto T, Igarashi T, Suzuki Y, Egawa S, Yoshimura N. Bladder wall injection of mesenchymal stem cells ameliorates bladder inflammation, overactivity, and nociception in a chemically induced interstitial cystitis-like rat model. Int Urogynecol J. 2018;29:1615–22. https://doi.org/10.1007/s00192-018-3592-8.
Dezawa M. Muse cells provide the pluripotency of mesenchymal stem cells: direct contribution of Muse cells to tissue regeneration. Cell Transplant. 2016;25:849–61. https://doi.org/10.3727/096368916X690881.
Kuroda Y, Kitada M, Wakao S, Nishikawa K, Tanimura Y, Makinoshima H, et al. Unique multipotent cells in adult human mesenchymal cell populations. Proc Natl Acad Sci U S A. 2010;107:8639–43. https://doi.org/10.1073/pnas.0911647107.
Kuroda Y, Kitada M, Wakao S, Dezawa M. Bone marrow mesenchymal cells: how do they contribute to tissue repair and are they really stem cells? Arch Immunol Ther Exp (Warsz). 2011;59:369–78. https://doi.org/10.1007/s00005-011-0139-9.
Yamada Y, Wakao S, Kushida Y, Minatoguchi S, Mikami A, Higashi K, et al. S1P-S1PR2 axis mediates homing of Muse cells into damaged heart for long-lasting tissue repair and functional recovery after acute myocardial infarction. Circ Res. 2018;122:1069–83. https://doi.org/10.1161/CIRCRESAHA.117.311648.
Iseki M, Kushida Y, Wakao S, Akimoto T, Mizuma M, Motoi F, et al. Muse cells, nontumorigenic pluripotent-like stem cells, have liver regeneration capacity through specific homing and cell replacement in a mouse model of liver fibrosis. Cell Transplant. 2017;26:821–40. https://doi.org/10.3727/096368916X693662.
Uchida N, Kushida Y, Kitada M, Wakao S, Kumagai N, Kuroda Y, et al. Beneficial effects of systemically administered human Muse cells in adriamycin nephropathy. J Am Soc Nephrol. 2017;28:2946–60. https://doi.org/10.1681/ASN.2016070775.
Shono Y, Kushida Y, Wakao S, Kuroda Y, Unno M, Kamei T, et al. Protection of liver sinusoids by intravenous administration of human Muse cells in a rat extra-small partial liver transplantation model. Am J Transplant. 2021;21:2025–39. https://doi.org/10.1111/ajt.16461.
Ozuru R, Wakao S, Tsuji T, Ohara N, Matsuba T, Amuran MY, et al. Rescue from Stx2-producing E. coli-associated encephalopathy by intravenous injection of Muse cells in NOD-SCID mice. Mol Ther. 2020;28:100–18. https://doi.org/10.1016/j.ymthe.2019.09.023.
Yabuki H, Wakao S, Kushida Y, Dezawa M, Okada Y. Human multilineage-differentiating stress-enduring cells exert pleiotropic effects to ameliorate acute lung ischemia-reperfusion injury in a rat model. Cell Transplant. 2018;27:979–93. https://doi.org/10.1177/0963689718761657.
Noda T, Nishigaki K, Minatoguchi S. Safety and efficacy of human Muse cell-based product for acute myocardial infarction in a first-in-human trial. Circ J. 2020;84:1189–92. https://doi.org/10.1253/circj.CJ-20-0307.
Fujita Y, Nohara T, Takashima S, Natsuga K, Adachi M, Yoshida K, et al. Intravenous allogeneic multilineage-differentiating stress-enduring cells in adults with dystrophic epidermolysis bullosa: a phase 1/2 open-label study. J Eur Acad Dermatol Venereol. 2021;35:e528–e31. https://doi.org/10.1111/jdv.17201.
Funahashi Y, Yoshida M, Yamamoto T, Majima T, Takai S, Gotoh M. Intravesical application of rebamipide promotes urothelial healing in a rat cystitis model. J Urol. 2014;192:1864–70. https://doi.org/10.1016/j.juro.2014.06.081.
Saitoh C, Yokoyama H, Chancellor MB, de Groat WC, Yoshimura N. Comparison of voiding function and nociceptive behavior in two rat models of cystitis induced by cyclophosphamide or acetone. Neurourol Urodyn. 2010;29:501–5. https://doi.org/10.1002/nau.20777.
Uchida H, Morita T, Niizuma K, Kushida Y, Kuroda Y, Wakao S, et al. Transplantation of unique subpopulation of fibroblasts, Muse cells, ameliorates experimental stroke possibly via robust neuronal differentiation. Stem Cells. 2016;34:160–73. https://doi.org/10.1002/stem.2206.
Fujita Y, Komatsu M, Lee SE, Kushida Y, Nakayama-Nishimura C, Matsumura W, et al. Intravenous injection of Muse cells as a potential therapeutic approach for epidermolysis bullosa. J Invest Dermatol. 2021;141:198–202.e6. https://doi.org/10.1016/j.jid.2020.05.092.
Kajitani T, Endo T, Iwabuchi N, Inoue T, Takahashi Y, Abe T, et al. Association of intravenous administration of human Muse cells with deficit amelioration in a rat model of spinal cord injury. J Neurosurg Spine. 2021;34(4):648–55. https://doi.org/10.3171/2020.7.SPINE20293.
Suzuki T, Sato Y, Kushida Y, Tsuji M, Wakao S, Ueda K, et al. Intravenously delivered multilineage-differentiating stress enduring cells dampen excessive glutamate metabolism and microglial activation in experimental perinatal hypoxic ischemic encephalopathy. J Cereb Blood Flow Metab. 2021;41:1707–20. https://doi.org/10.1177/0271678X20972656.
Yamashita T, Kushida Y, Wakao S, Tadokoro K, Nomura E, Omote Y, et al. Therapeutic benefit of Muse cells in a mouse model of amyotrophic lateral sclerosis. Sci Rep. 2020;10:17102. https://doi.org/10.1038/s41598-020-74216-4.
Furuta A, Jankowski RJ, Pruchnic R, Yoshimura N, Chancellor MB. The potential of muscle-derived stem cells for stress urinary incontinence. Expert Opin Biol Ther. 2007;7:1483–6. https://doi.org/10.1517/14712598.7.10.1483.
Kim A, Shin DM, Choo MS. Stem cell therapy for interstitial cystitis/bladder pain syndrome. Curr Urol Rep. 2016;17:1. https://doi.org/10.1007/s11934-015-0563-1.
Acknowledgements
The authors would like to thank Chieko Yoshimoto and Atsuko Sasaki, who are research technicians at the department of Urology, Jikei University School of Medicine. This study was supported by JSPS KAKENHI Grant Number JP21K09382.
Author information
Authors and Affiliations
Contributions
A. Furuta: protocol, data collection, manuscript writing; Y. Kuroda: data collection; T. Yamamoto: management data analysis; S. Egawa: manuscript editing; M. Dezawa: manuscript editing; N. Yoshimura: protocol, management data analysis, manuscript editing.
Corresponding author
Ethics declarations
Financial disclaimers/conflicts of interest
This study was supported by Life Science Institute, Inc.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Furuta, A., Kuroda, Y., Yamamoto, T. et al. Effects of human Muse cells on bladder inflammation, overactivity, and nociception in a chemically induced Hunner-type interstitial cystitis-like rat model. Int Urogynecol J 33, 1293–1301 (2022). https://doi.org/10.1007/s00192-022-05166-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00192-022-05166-w