Abstract
The objective was to assess the effectiveness of supportive underwear (Pro-Portare®) in women with pelvic organ prolapse. Symptomatic women with all stages of prolapse participated in the study and were followed up for 4 weeks. The effectiveness of Pro-Portare® was measured by three validated disease-specific quality-of-life questionnaires, a daily diary and a questionnaire on comfort and effectiveness. Thirteen women were included in the study. No improvement on quality of life was found with the use of Pro-Portare®. None of the women regarded Pro-Portare® as very effective, whereas five women regarded the device as (reasonably) effective. The women’s major concern was on the size, shape and hard consistency of the inlet. Two women experienced worsening of their prolapse. Pro-Portare® was not effective in the alleviation of prolapse symptoms and was no final solution in any of the women.
Similar content being viewed by others
Introduction
Pelvic organ prolapse is a major health care problem. Eleven percent of women will undergo surgical pelvic reconstructive therapy during a lifetime, and surgery is considered the most effective therapy [1]. However, recurrence rates are high, and surgery is not feasible in all patients. Currently, the only other viable option is pessary treatment. Pessaries are considered safe and easy to use. Prospective observational studies show success rates between 60% and 80% [2], which decline with increasing parity and the duration of pessary use [3, 4]. In some women, however, pessary use is not feasible due to pain, erosion or repeated loss of the pessary [3, 4]. Recently, a medical device has been developed for use in these patients with pelvic organ prolapse. Pro-Portare® provides support to the pelvic floor and prevents the descent of the pelvic organs outside the vagina as well as symptomatic descent of the pelvic floor itself (descending perineum).
At present, the effectiveness of any kind of supportive underwear in women with symptoms of pelvic organ prolapse is unknown, and there are no scientific data available up till now. Our hypothesis is that Pro-Portare® has a positive effect on the quality of life in women with pelvic organ prolapse.
Materials and methods
This prospective observational study was conducted in a tertiary referral urogynaecological clinic at the Radboud University Nijmegen Medical Centre, The Netherlands, from January till December 2006. The local ethics committee has approved the study, and all participants gave their informed consent.
All patients with pelvic organ prolapse quantification (POPQ) stage, who were either awaiting surgical correction or were not eligible for surgical or pessary therapy, were asked to participate in the study. The exclusion criterion was the inability to speak the Dutch language. The patient’s selection was not consecutive but depended mainly on the availability of the researchers.
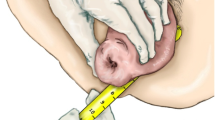
The supportive underwear consisted of tricot-knitted knickers with Velcro in the waist and a silicone inlet that supports the pelvic floor and the prolapse (Figs. 1 and 2).
Verbal and written information on the use of Pro-Portare® was provided. The women were given two sets of Pro-Portare® on loan for 4 weeks, and they were asked to wear Pro-Portare® for these 4 weeks. The questionnaires Urogenital Distress Inventory (UDI), Incontinence Impact Questionnaire (IIQ) and Defecatory Distress Inventory (DDI) were completed at baseline and at the end of the study period [5]. The UDI consists of 11 items and five subscales on bothersome urinary complaints. The IIQ consists of 13 items and measures the impact of urinary incontinence on quality of life in five subscales. The DDI measures bothersome defecatory complaints and consists of ten items and four subscales. The score on each subscale of these questionnaires ranges from 0–100, where 0 indicates the best quality of life and 100 indicates the poorest quality of life.
The women kept a daily diary. The diary was introduced with a statement that the aim was to report on their physical activities and their findings in relation to the supportive underwear. The number of days the women had worn the supportive underwear were recorded in the diary.
Furthermore, a self-developed questionnaire on the patients’ perception of the comfort and the effectiveness of Pro-Portare® was completed at the end of the study period. The following questions were asked:
-
1.
What is your overall impression on the comfort of Pro-Portare®?
-
2.
What is your opinion on the effectiveness of Pro-Portare®?
The possible answers to the first question were good, neutral and poor. The possible answers to the second question were very effective, effective, reasonably effective, not effective and worsening of symptoms. In case the women noted worsening of symptoms, clinical examination (POPQ) was repeated at the end of the study period.
The women completed the UDI, IIQ and DDI at baseline, which was prior to the use of Pro-Portare®. The second measurement was ideally after 4 weeks. In case a woman stopped using Pro-Portare® before the end of the study period of 4 weeks, however, the UDI, IIQ and DDI as well as the questionnaire on the comfort and effectiveness were completed at that timepoint.
Differences within the group (at baseline and the end of the study period) were tested for statistical significance using the Wilcoxon signed-rank test for two related samples. Software package Statistical Package for the Social Sciences 13.0 (SPSS, Chicago, IL, USA) was used for statistical analysis. p values <0.05 were considered statistically significant.
Results
Thirteen women were included in this study, with POPQ stages 1–4 prolapse [three women (23%), stage 1; four women (31%), stage 2; four women (31%), stage 3; and two women (15%), stage 4). Their median age was 67 years (range, 49–85 years), and the median body mass index was 25.1 kg/m2 (range, 22.1–36.3 kg/m2). All women had symptoms of pelvic organ prolapse, of which four women (31%) had previously undergone prolapse surgery. Four women (31%) were on the surgical waiting list for prolapse surgery during the study period, whereas another six women (46%) underwent surgical prolapse correction within 6 months after completion of the study. The remaining three women (23%) were not eligible for surgery. Seven women (56%) reported symptoms of urinary incontinence more than once a week. Three women (23%) had pelvic floor physiotherapy in the period immediately prior to the study but not during the study period. Three out of 12 post-menopausal women (25%) were on local estrogen therapy or hormonal replacement therapy.
The median number of days until the end of the study period was 11 days (range, 1–28 days). Only two women have worn the Pro-Portare® during the entire 4-week period.
Twelve women (92%) completed the questionnaires UDI, IIQ and DDI at both timepoints. The results were as shown in Table 1. The differences between the baseline measurement and measurement at the end of the study period were not statistically significant.
Nine women (69%) completed the daily diary and the questionnaire on their findings with respect to comfort and effectiveness. Three women (33%) considered the comfort of Pro-Portare® as good and two women (22%) as poor. The other four women (44%) answered neutral. Regarding the effectiveness of Pro-Portare®, none of the women regarded the device as very effective, whereas five women considered the device either effective (22%) or reasonably effective (33%). Two women (22%) stated that the supportive underwear was not effective, and another two women (22%) experienced worsening of the prolapse symptoms. In these two women, the worsening was also seen on clinical examination (POPQ point Ba from +1 to +3 cm and from +1 to +2 cm at baseline and after 4 weeks, respectively).
The women’s perceptions as stated in the diary were as follows: The silicone inlet was experienced as too hard (five women, 56%), too large (three women, 33%) and too stiff (one woman, 11%). Consequently, the underwear was getting visible under normal clothes and caused some problems with skin irritation on the inside of the legs. The Pro-Portare® was considered comfortable during walking, housekeeping and shopping, whereas sitting on a hard underground or riding a bicycle was generally considered uncomfortable.
One woman continued to wear Pro-Portare® for 6 months after the study period and subsequently underwent prolapse surgery due to progressive signs and symptoms of prolapse.
Discussion
In this pilot study, we have evaluated the effectiveness of supportive underwear (Pro-Portare®) in 13 women with pelvic organ prolapse. There was no improvement in quality of life with the use of Pro-Portare®, and none of the women has used Pro-Portare® beyond the time period of 6 months. To our knowledge, this is the first report on the effectiveness of supportive underwear in women with pelvic organ prolapse. As compared with data on surgical therapy and pessaries, Pro-Portare® seems to be a less effective treatment option [1, 2].
In this study, half of the women had a POPQ stage 1 or 2 prolapse. Especially, a stage 1 prolapse is generally not regarded as a pathological finding. The POPQ and other staging systems, however, disregard the descent of the perineum or pelvic floor. Due to the nature of our clinic, which is a tertiary referral centre, women with symptoms but no signs of prolapse are probably slightly overrepresented. We were not able, however, to demonstrate a difference in effectiveness of Pro-Portare® in these women.
Two women (15%) have noted that their prolapse had worsened during the study period. This worsening was seen on clinical examination as well. However, not all women in the study underwent prolapse staging at the end of the study period. It is not known whether this worsening is a side effect of the Pro-Portare® or just represents the natural course of prolapse. This needs to be investigated in future studies on supportive underwear in women with prolapse.
Pro-Portare® is not yet registered as a medical device and is thus not covered by the Dutch health insurance companies. Pro-Portare®, as a set of two pieces of underwear and one silicone inlet, costs approximately 300 € (US $200). Only one size of Pro-Portare® is available until now. The hard consistency and the size of the inlet have caused discomfort to the women in the study. Whether a thinner version of the silicone inlet with more souplesse would be more effective needs to be awaited. Further development of the device in various sizes is pending.
An obvious limitation of this study is its small sample size. Moreover, the results from this small pilot study are rather negative. In all likelihood, however, these results would not change if more women would have been included. To our opinion, it is of utmost importance that negative results are also published and are appreciated by gynaecologists in the field. To what extent the lack of effectiveness is due to the lack of comfort of the device as used in the study is, however, not known until now.
In conclusion, Pro-Portare® did not change the quality of life in the women included in this study. Furthermore, Pro-Portare® was no final solution in any of these women. Although there are only few treatment options for women with pelvic organ prolapse, at this stage, broad implementation of supportive underwear is not very likely.
Abbreviations
- POP:
-
pelvic organ prolapse
- POPQ:
-
pelvic organ prolapsed quantification
- UDI:
-
Urogenital Distress Inventory
- DDI:
-
Defecatory Distress Inventory
- IIQ:
-
Incontinence Impact Questionnaire
- p :
-
p value for differences within the group using Wilcoxon signed-rank test for two related samples
References
Olsen AL, Smith VJ, Bergstrom JO, Colling JC, Clark AL (1997) Epidemiology of surgically managed pelvic organ prolapse and urinary incontinence. Obstet Gynecol 89(4):501–506
Hanson LM, Schulz JA, Flood CG, Cooley B, Tam F (2006) Vaginal pessaries in managing women with pelvic organ prolapse and urinary incontinence: patient characteristics and factors contributing to success. Int Urogynecol J 17:155–159
Vierhout ME (2004) The use of pessaries in vaginal prolapse. Eur J Obstet Gynecol Reprod Biol 117(1):4–9
Fernando RJ, Thakar R, Sultan AH, Shah SM, Jones PW (2006) Effect of vaginal pessaries on symptoms associated with pelvic organ prolapse. Obstet Gynecol 108(1):93–99
van der Vaart CH, de Leeuw JR, Roovers JP, Heintz AP (2003) Measuring health-related quality of life in women with urogenital dysfunction: the urogenital distress inventory and incontinence impact questionnaire revisited. Neurourol Urodyn 22(2):97–104
Conflicts of interest
None.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This is an open access article distributed under the terms of the Creative Commons Attribution Noncommercial License (https://creativecommons.org/licenses/by-nc/2.0), which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
About this article
Cite this article
Lammers, K., Vierhout, M.E., Withagen, M.I.J. et al. The effectiveness of supportive underwear in women with pelvic organ prolapse: a pilot study. Int Urogynecol J 19, 1519–1522 (2008). https://doi.org/10.1007/s00192-008-0676-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00192-008-0676-x