Abstract
Purpose
Ilofotase alfa is a human recombinant alkaline phosphatase with reno-protective effects that showed improved survival and reduced Major Adverse Kidney Events by 90 days (MAKE90) in sepsis-associated acute kidney injury (SA-AKI) patients. REVIVAL, was a phase-3 trial conducted to confirm its efficacy and safety.
Methods
In this international double-blinded randomized-controlled trial, SA-AKI patients were enrolled < 72 h on vasopressor and < 24 h of AKI. The primary endpoint was 28-day all-cause mortality. The main secondary endpoint was MAKE90, other secondary endpoints were (i) days alive and free of organ support through day 28, (ii) days alive and out of the intensive care unit (ICU) through day 28, and (iii) time to death through day 90. Prior to unblinding, the statistical analysis plan was amended, including an updated MAKE90 definition.
Results
Six hundred fifty patients were treated and analyzed for safety; and 649 for efficacy data (ilofotase alfa n = 330; placebo n = 319). The observed mortality rates in the ilofotase alfa and placebo groups were 27.9% and 27.9% at 28 days, and 33.9% and 34.8% at 90 days. The trial was stopped for futility on the primary endpoint. The observed proportion of patients with MAKE90A and MAKE90B were 56.7% and 37.4% in the ilofotase alfa group vs. 64.6% and 42.8% in the placebo group. Median [interquartile range (IQR)] days alive and free of organ support were 17 [0–24] and 14 [0–24], number of days alive and discharged from the ICU through day 28 were 15 [0–22] and 10 [0–22] in the ilofotase alfa and placebo groups, respectively. Adverse events were reported in 67.9% and 75% patients in the ilofotase and placebo group.
Conclusion
Among critically ill patients with SA-AKI, ilofotase alfa did not improve day 28 survival. There may, however, be reduced MAKE90 events. No safety concerns were identified.
Similar content being viewed by others
Sepsis-associated acute kidney injury in patients admitted to an intensive care unit is associated with significant morbidity and mortality. There is currently no pharmaceutical treatment. Although we found no evidence that ilofotase alfa improved survival, it may reduce major adverse kidney events (mortality, new onset, renal replacement therapy >25% reduction in estimated glomerular filtration rate, or rehospitalization) up to 90 days. |
Introduction
Sepsis is the leading cause of acute kidney injury (AKI) in critically ill patients as a consequence of inflammatory, direct nephrotoxic, and ischemic processes [2,3,4,5]. Development of AKI during sepsis (SA-AKI) is independently associated with increased morbidity and mortality [6, 7]. Patients with SA‐AKI are at risk of developing chronic kidney disease (CKD) resulting in a considerable burden for patients and society [8]. Conversely, underlying CKD markedly increases the risk of AKI and the risk increases proportionally with severity of CKD [9]. Pre-existent CKD complicated by AKI is common in critically ill patients and associated with delayed kidney function recovery and increased risk of rehospitalization and development of end-stage renal disease [10].
There are no pharmacological therapies approved for the treatment of SA-AKI. Management consists of only secondary prevention and supportive care strategies, such as fluids and renal replacement therapy (RRT) [6, 11]. Alkaline phosphatase (ALP) is an endogenous detoxifying enzyme that plays a significant role in host defense and innate immunity, particularly acting as an endogenous anti-inflammatory protein [12, 13]. For example, removal of one of two phosphate groups abolishes the biological activity of endotoxin; the dephosphorylated endotoxin acts as a toll-like receptor 4 (TLR4) antagonist [14]. Furthermore, ALP dephosphorylates extracellular adenosine triphosphate (ATP), that is pro-inflammatory, resulting in generation of adenosine, which has anti-inflammatory and tissue-protective effects. In particular, the kidney is negatively affected by increased levels of ATP, while adenosine has reno-protective effects [15]. Hence, dephosphorylation attenuates the inflammatory response and exerts tissue-protective properties [16]. In animal models of sepsis, ALP administration dampens inflammation and reduces mortality [13, 17], but also protects against ischemia–reperfusion injury [18,19,20].
To augment therapeutic efficacy, human recombinant ALP, named ilofotase alfa, was developed, consisting of an intestinal ALP sequence (highest biological activity) and a crown domain corresponding with placental ALP sequence (to enhance stability) [21]. Dephosphorylation properties of ilofotase alfa were confirmed [21] and considered to reduce systemic and local inflammation and attenuate organ damage [15]. Two small phase-2 studies with bovine ALP [16, 22] demonstrated reduced urinary detection of tubular injury markers and more pronounced improvement of endogenous creatinine clearance. In addition to renal protective effects, a survival benefit was observed as a secondary endpoint in a large phase-2 trial [23]. The REVIVAL study aimed to confirm the effect of ilofotase alfa on 28-day all-cause mortality in critically ill patients with SA-AKI; Major Adverse Kidney Events by 90 days (MAKE90) were the main secondary endpoint.
Methods
Ethics and dissemination
This trial was conducted in accordance with the protocol and consensus ethical principles of international guidelines including the Declaration of Helsinki, Council for International Organizations of Medical Sciences (CIOMS) International Ethical Guidelines, and International Conference on Harmonization (ICH) Good Clinical Practice (GCP) Guidelines. The protocol, the single substantial protocol amendment, and other relevant documents were reviewed and approved by the Institutional Research Board (IRB)/Institutional Ethics Committee (IEC) in the relevant centers prior to being used in the trial. Informed consent was obtained from all patients or the patient’s legal representative.
Trial design
This was a phase-3, multi-center, randomized, double-blind, placebo-controlled, two-arm parallel-group-sequential design trial in which patients with SA-AKI were randomly assigned in a 1:1 ratio to ilofotase alfa or matching placebo in Europe, North America, Australia, New Zealand, and Japan. The maximum sample size of 1400 patients in the main trial population provided ~ 85% power assuming a 35% mortality rate in the placebo group and an 8% absolute treatment effect. A one-sided p value < 0.025 for superiority was considered to indicate statistical significance. For the first interim analysis (N = 400 patients), a < 15% estimated power to demonstrate a significant effect at full enrollment (1400 patients), was defined as non-binding threshold to stop early for futility.
Patients, randomization, and study medication
The population studied were adult patients with sepsis and recent onset AKI requiring vasopressor support. The complete inclusion and exclusion criteria are described in supplemental Table 1. Full details on population and study design (including the protocol amendment) and conduct were previously published [1]. Briefly, three patient cohorts were defined: (i) patients with SA-AKI with a pre-AKI reference estimated glomerular filtration rate (eGFR) ≥ 45 mL/min/1.73 m2 and no proven or suspected coronavirus disease 2019 (COVID-19) at time of randomization (‘main trial population’); (ii) patients with a pre-AKI reference eGFR ≥ 25 and < 45 mL/min/1.73 m2 (‘moderate-to-severe CKD population or mCKD’) with sepsis and AKI and no proven or suspected COVID-19 at time of randomization; (iii) patients with COVID-19-induced sepsis and AKI at time of randomization (‘COVID-19 population’). The ‘all combined population’ includes all three cohorts. The randomization schedule was stratified by site and modified Sequential Organ Failure Assessment (mSOFA) (≤ 9, > 9), excluding the neurological component of SOFA. An independent statistician generated a permuted block randomization schedule (block size of 4) for an interactive voice/web response system, which linked sequential patient randomization numbers to treatment codes.
Study medication was administered, within 24 h if sepsis was present prior to AKI or within 48 h if AKI was present when sepsis was diagnosed, at 1.6 mg (1000 U) per kg of patient body weight up to 120 kg, with a fixed dose of 192 mg in patients > 120 kg [1]. Patients received study medication as a 1-h infusion once daily for 3 consecutive days. All personnel involved in this study were blinded to treatment assignment and clinicians were not allowed to measure serum ALP concentrations until day 14 [24]. The trial drug was provided in addition to usual care as outlined in the Surviving Sepsis Campaign guidelines [25] and Kidney Disease Improving Global Outcomes (KDIGO) guidelines [6]. Initiation and termination of RRT were based on conventional criteria [26,27,28]. A Data Management Committee (DMC) was installed and provided with stopping rules based on pre-defined threshold for futility or early success.
Primary endpoint
The primary efficacy endpoint was 28-day all-cause mortality.
Secondary endpoints
Renal endpoints
The main secondary endpoint was MAKE90. Two definitions of MAKE90 were stipulated in the amended Statistical Analysis Plan (SAP) [1]. MAKE90A included mortality through day 90, or an eGFR drop of > 25% at day 90 compared to pre-AKI value, or any RRT events through day 28 or RRT status at day 90 or rehospitalization. Rehospitalization was added to bridge to the results of the phase-2 STOP-AKI study and defined as any overnight stay in a hospital after initial discharge from the hospital. MAKE90B, based on mortality through day 90 or an eGFR drop of > 25% at day 28 and day 90, or need for RRT at day 90. Furthermore, as post hoc analysis, the impact of prior renal function (pre-AKI eGFR) on MAKE90 was examined.
Other secondary endpoints
Additional secondary endpoints were days alive and free of organ support; days alive and discharged from intensive care unit (ICU) through day 28; and time to death through day 90.
Safety endpoints
Safety endpoints and adverse events (AEs) were monitored until study day 28 (inclusive). All deaths were recorded up to study day 180.
Data analyses and statistics
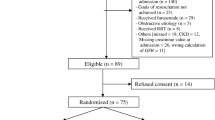
Analysis of the primary efficacy endpoint was conducted according to protocol and SAP based on the modified intention to treat (mITT) population, defined as all patients in whom drug administration was started and analyzed according to treatment allocation. The original SAP was amended prior to unblinding and any analysis of the secondary endpoints to prioritize analysis of the all combined population [1] for hypothesis generation. The combined population consisted of all patients allocated to treatment group according to received treatments including patients allocated to main/moderate CKD subpopulation according to the reported pre-AKI-reference eGFR value (see Fig. 1). Safety analyses were conducted in the safety population.
For categorical and binary demographic and baseline characteristics, absolute and relative frequencies are presented; and for continuous variables, the median and interquartile ranges (IQR) are presented.
For the endpoints 28-day all-cause mortality, 90-day all-cause mortality, MAKE90A and MAKE90B, observed proportions are presented along with the difference in proportions (i.e., ilofotase alfa minus placebo), the 95% confidence interval (CI) for the difference in proportions and the one-sided nominal p value based on a z test. Logistic regression was used as a sensitivity analysis to take account of the separate cohorts in the population, for 28-day mortality, MAKE90A and MAKE90B endpoints. For days alive and free of organ support and days alive and out of ICU (both until day 28), descriptive measures as well as one-sided nominal p values are reported. In addition, in a post hoc analysis for each treatment group, the effect of pre-AKI eGFR on the predicted probability of a MAKE90A outcome was investigated using a logistic regression model which included treatment group, pre-AKI eGFR, and an interaction term for treatment group by pre-AKI eGFR.
Safety analyses were conducted in the safety population involving all patients enrolled in the study randomized to a treatment arm. For patients with AEs, relative and absolute frequencies are presented, and the distribution of the number of patients with (serious) AEs has been compared using the chi-squared test.
SAS (SAS software version 9.4; SAS Institute Inc., Cary, NC, USA) was used for all statistical analyses. A one-sided p value < 0.025 for superiority was considered to indicate statistical significance. In case the primary was not met, further statistical testing was considered exploratory and no correction for multiple testing was applied.
Results
Patient recruitment and demographic characteristics
From November 2020 to July 2022, 649 patients (main [n = 567] CKD [n = 49] and COVID-19 [n = 33] cohorts) were enrolled and treated in 107 sites in North America, Europe, Japan, and Oceania (Fig. 1) and included in the analyses. Demographic characteristics are presented in Table 1 (demographics per cohort in supplementary Table 2). Within all three cohorts, patient demographics were comparable between the ilofotase alfa and placebo group.
Interim analysis
At the time of the first interim analysis on 1st July 2022 (based on 411 patients in the main trial mITT population), all-cause 28-day mortality was 61/208 (29.3%) and 52/203 (25.7%) for the ilofotase alfa and placebo groups, resulting in a predictive probability of success of 1.2%. The non-binding recommendation from the DMC was to stop the REVIVAL trial early based on futility according to the pre-defined threshold of < 15% predictive probability of success. There were no safety concerns.
Mortality
For the combined population (including main, CKD, COVID-19 cohorts), there was no difference in mortality between the ilofotase alfa (92 of 330 patients) and the placebo (89 of 319 patients) groups at day 28 (27.9% vs 27.9%, respectively). The difference in proportions was − 0.02% with a 95% CI of [− 6.9%; 6.9%] and a nominal one-sided p value of 0.50. Day 90 mortality was 112 of 330 patients (33.9%) vs 111 of 319 patients (34.8%). Mortality data per cohort are presented in supplementary Table 3. The treatment difference taking account of the three cohorts, estimated based on logistic regression, gave an odds ratio of 0.99 (95% CI (0.70–1.40), nominal one-sided p = 0.47).
Renal endpoints
The difference in observed proportions of patients with a MAKE90A event between ilofotase alfa (56.7%, 187 of 330) and the placebo group (64.6%, 206 of 319) groups was 7.9%, 95% CI [− 15.4%, − 0.4%] with a nominal one-sided p value of 0.02. This effect was predominately driven by the difference in receipt of RRT through day 90 between the ilofotase alfa and placebo groups (28.2% vs 36.4%) (Table 2).
There was no difference in observed proportions of patients with a MAKE90B event between ilofotase alfa (36.4%, 120 of 330) and the placebo (40.1%, 128 of 319) groups (difference − 3.8% 95% CI [− 11.2%, 3.7%] with a nominal one-sided p value of 0.16). Estimated proportions of patients with a MAKE90A or MAKE90B event are provided in supplementary Table 4. The treatment difference taking account of the three cohorts, estimated based on logistic regression, resulted in an odds ratio of 0.72 (95% CI (0.52–0.99), nominal one-sided p = 0.022) for MAKE90A and an odds ratio of 0.84 (95% CI (0.61–1.16), nominal one-sided p = 0.15) for MAKE90B.
In a post hoc analysis, there was evidence of an interaction between renal function prior to the SA-AKI episode (pre-AKI eGFR) and the incidence of the MAKE90A event, suggesting that the therapeutic efficacy of ilofotase alfa was more pronounced in patients with a lower pre-AKI eGFR, i.e., more severe pre-existent CKD (p = 0.024, Fig. 2). Ilofotase alfa showed a benefit over placebo in the probability of an MAKE90A event for patients with pre-AKI eGFR below approximately 90 mL/min/1.73 m2. At the lower quartile of 59.8 mL/min/1.73 m2 for pre-AKI eGFR, the probability of an MAKE90A event was predicted to be 71% for a placebo patient versus 57% for an ilofotase alfa patient. At the upper quartile of 89 mL/min/1.73m2, this difference had virtually disappeared and the respective probabilities were 58% and 56%.
Predicted probability of an MAKE90A event with 95% confidence limits. Predicted probabilities are taken from the results of the logistic regression model which included “MAKE through day 90” as the outcome of interest, and treatment, pre-AKI reference eGFR, and pre-AKI reference eGFR by treatment interaction (p for interaction = 0.0235). Patients who did not meet the criteria for having a confirmed “MAKE through day 90” event are assumed to not have a “MAKE through day 90” event in the logistic regression model
Other secondary endpoints
There were no significant differences in the use of organ support, as median [IQR] days alive and free of organ support were 17 [0–24] and 14 [0–24] days for ilofotase alfa and placebo groups (nominal p value 0.22). Number of days alive and discharged from the ICU through day 28 tended to be higher in the ilofotase alfa group 15 [0–22] days compared to 10 [0–22] days in the placebo group, but this difference did not reach statistical significance (nominal p value 0.09) (Table 2). Other secondary endpoints per patient cohort are depicted in supplementary Table 5.
Safety parameters
Reported AEs are summarized by occurrence, percentage of occurrence, severity, seriousness, outcome, and relation to treatment in the safety population (Table 3). A lower number of patients experienced any AEs in the ilofotase alfa group compared to the placebo group [224/330 (67.9%) in the ilofotase alfa group and 240/320 (75%) in the placebo group with a nominal two-sided p value based on the chi-squared test of 0.045]. Overall, the incidence of serious AEs was similar between the two treatment groups. There were no relevant differences observed on SOC (System Organ Class Level) (supplementary Table 6).
Discussion
Given the observation that AKI complicates sepsis and patients with AKI have far worse short- and long-term outcomes, there is high interest in strategies that reduce the incidence or ameliorate the course of AKI, with the goal of reducing the overall burden on patients. In this phase-3 multi-center, international double-blind randomized controlled trial ilofotase alfa did not decrease 28-day all-cause mortality. However, there was evidence to suggest ilofotase alfa reduced MAKE90 events, mainly driven by lowering the incidence of RRT through day 90 in these patients. Ilofotase alfa was well tolerated, and no safety issues emerged.
In the previous 300-patient phase-2 trial, day-28 mortality was 14% in the treatment group compared to 27% in the placebo group [23]. This observed survival benefit of ilofotase alfa [23] was not confirmed in the current trial and several reasons for this discrepancy with the previous trial in the observed effect of ilofotase alfa on survival can be put forward. First, the drug might not improve survival and the effect observed in the previous phase-2 STOP-AKI trial was a false-positive type 1 error. Second, slight changes in eligibility criteria between both trials could be responsible. For example, in the REVIVAL trial, patients who already had AKI when they presented with sepsis were eligible, in contrast to the STOP-AKI trial. It is plausible that the duration of AKI was longer in these patients, possibly limiting the therapeutic efficacy of ilofotase alfa. It is also plausible that other differences in phenotype between the STOP-AKI and REVIVAL patients existed that may have been of relevance, e.g., due to an overall change in the ICU population, because of the COVID-19 pandemic and general heterogeneity in the sepsis population. Indeed, we found the evidence of heterogeneity of treatment effect with a more marked reduction in MAKE90 events in patients with pre-existent impaired renal function. Third, REVIVAL was conducted in 107 sites worldwide vs 55 sites in STOP-AKI, which increases generalizability but may have also negatively influenced therapeutic efficacy.
Although the trial was stopped early because of the lack of an apparent survival benefit, ilofotase alfa did reduce MAKE90 events, which is consistent with previous trial results. Earlier clinical data showed that bovine ALP attenuated urinary detection of tubular injury marker Glutathione S-transferase A1 (GSTA1-1), improved creatinine clearance and reduced the need for RRT [16]. Subsequently, a human recombinant Alkaline Phosphatase (ilofotase alfa) was developed and tested in a phase-2 trial in 301 patients with SA-AKI. Although the primary endpoint in this trial, improvement of endogenous creatinine clearance (ECC) in 7 days, was not met, longer term ECC over the 28 day study period was significantly better, and in addition to the described survival benefit, a reduction in MAKE90 events was observed with ilofotase alfa (26%) compared to placebo (40%) [23]. In REVIVAL, MAKE90A was also markedly reduced in the ilofotase alfa treatment arm (57% versus 65% in the placebo arm), mainly driven by lower incidence of RRT through day 90. Need for RRT is an important component of this composite endpoint especially due to the strong association with poor outcomes in patients with sepsis [29,30,31,32]. Furthermore, it is recognized that patients with pre-existent CKD are more likely to suffer from AKI when they develop sepsis and have a higher risk of not recovering renal function [9, 33]. It is, therefore, not surprising that patients with a pre-existent lower eGFR, i.e., reduced or no renal reserve, are more likely to meet a MAKE90 criterion following sepsis and that the reno-protective effect observed with ilofotase alfa in REVIVAL was more pronounced in these patients. Increased efficacy in patients with pre-existent CKD has been observed with other interventions as well [9, 27]. As pre-existent renal function was not collected in the previous (recombinant) alkaline phosphatase trials, the interplay between pre-existent renal function and therapeutic efficacy of ilofotase alfa is a novel finding that emerged from post hoc analysis, and should be interpreted as a hypothesis generating finding that needs to be confirmed.
Strengths of this study include its generalizability, as it was conducted in multiple countries worldwide. Second, the group sequential trial design allowed for the results to be reported in case the trial was terminated prematurely for futility. The latter also relates to two limitations. First, in case the primary endpoint was not met, further statistical testing was considered explorative and, therefore, no correction for multiple testing was performed; and second, analyses of combined cohorts were reported to explore trends based on all enrolled patients. The number of patients enrolled in the COVID-19 and CKD cohorts were small, and depicted in supplemental files for transparency. Further randomized-controlled trials would be necessary to assess the reno-protective effects of ilofotase alfa.
Conclusions
The REVIVAL trial was stopped for futility due to lack of evidence of reduced 28-day mortality with ilofotase alfa treatment in critically ill patients with SA-AKI. Despite this, our findings were consistent with preclinical studies and phase-2 trials suggesting potential benefit of ilofotase alfa in lowering MAKE90 events, mainly driven by an attenuated incidence of RRT through day 90. The reduction in MAKE90 with ilofotase alfa appeared most pronounced in patients with a lower eGFR prior to the SA-AKI episode. A prospective randomized-controlled trial is warranted to confirm the beneficial renal effects of ilofotase alfa in patients with SA-AKI and pre-existing CKD.
Data availability
Data is available upon reasonable request.
Change history
04 March 2024
A Correction to this paper has been published: https://doi.org/10.1007/s00134-024-07357-z
References
Pickkers P, Angus DC, Arend J, Bellomo R, van den Berg E, Bernholz J, Bestle M, Broglio K, Carlsen J, Doig CJ, Ferrer R, Joannidis M, Francois B, Doi K, Kellum JA, Laterre PF, Liu K, Mehta RL, Murray PT, Ostermann M, Pettilä V, Richards S, Young P, Zarbock A, Kjølbye AL (2023) Study protocol of a randomised, double-blind, placebo-controlled, two-arm parallel-group, multi-centre phase 3 pivotal trial to investigate the efficacy and safety of recombinant human alkaline phosphatase for treatment of patients with sepsis-associated acute kidney injury. BMJ Open 13:e065613
Wen X, Peng Z, Kellum JA (2011) Pathogenesis of acute kidney injury: effects of remote tissue damage on the kidney. Contrib Nephrol 174:129–137
Gomez H, Ince C, De Backer D, Pickkers P, Payen D, Hotchkiss J, Kellum JA (2014) A unified theory of sepsis-induced acute kidney injury: inflammation, microcirculatory dysfunction, bioenergetics, and the tubular cell adaptation to injury. Shock 41:3–11
Verma SK, Molitoris BA (2015) Renal endothelial injury and microvascular dysfunction in acute kidney injury. Semin Nephrol 35:96–107
Zarbock A, Nadim MK, Pickkers P, Gomez H, Bell S, Joannidis M, Kashani K, Koyner JL, Pannu N, Meersch M, Reis T, Rimmelé T, Bagshaw SM, Bellomo R, Cantaluppi V, Deep A, De Rosa S, Perez-Fernandez X, Husain-Syed F, Kane-Gill SL, Kelly Y, Mehta RL, Murray PT, Ostermann M, Prowle J, Ricci Z, See EJ, Schneider A, Soranno DE, Tolwani A, Villa G, Ronco C, Forni LG (2023) Sepsis-associated acute kidney injury: consensus report of the 28th Acute Disease Quality Initiative workgroup. Nat Rev Nephrol 19:401–417
Kellum JA, Lameire N (2013) Diagnosis, evaluation, and management of acute kidney injury: a KDIGO summary (part 1). Crit Care 17:204
Kellum JA, Chawla LS, Keener C, Singbartl K, Palevsky PM, Pike FL, Yealy DM, Huang DT, Angus DC (2016) The effects of alternative resuscitation strategies on acute kidney injury in patients with septic shock. Am J Respir Crit Care Med 193:281–287
Chawla LS, Eggers PW, Star RA, Kimmel PL (2014) Acute kidney injury and chronic kidney disease as interconnected syndromes. N Engl J Med 371:58–66
Khosla N, Soroko SB, Chertow GM, Himmelfarb J, Ikizler TA, Paganini E, Mehta RL (2009) Preexisting chronic kidney disease: a potential for improved outcomes from acute kidney injury. Clin J Am Soc Nephrol 4:1914–1919
Abdalrahim MS, Khalil AA, Alramly M, Alshlool KN, Abed MA, Moser DK (2020) Pre-existing chronic kidney disease and acute kidney injury among critically ill patients. Heart Lung 49:626–629
Kellum JA, Prowle JR (2018) Paradigms of acute kidney injury in the intensive care setting. Nat Rev Nephrol 14:217–230
Verweij WR, Bentala H, Huizinga-van der Vlag A, Miek van Loenen-Weemaes A, Kooi K, Meijer DK, Poelstra K (2004) Protection against an Escherichia coli-induced sepsis by alkaline phosphatase in mice. Shock 22:174–179
Su F, Brands R, Wang Z, Verdant C, Bruhn A, Cai Y, Raaben W, Wulferink M, Vincent JL (2006) Beneficial effects of alkaline phosphatase in septic shock. Crit Care Med 34:2182–2187
Wy CA, Goto M, Young RI, Myers TF, Muraskas J (2000) Prophylactic treatment of endotoxic shock with monophosphoryl lipid A in newborn rats. Biol Neonate 77:191–195
Peters E, Geraci S, Heemskerk S, Wilmer MJ, Bilos A, Kraenzlin B, Gretz N, Pickkers P, Masereeuw R (2015) Alkaline phosphatase protects against renal inflammation through dephosphorylation of lipopolysaccharide and adenosine triphosphate. Br J Pharmacol 172:4932–4945
Peters E, van Elsas A, Heemskerk S, Jonk L, van der Hoeven J, Arend J, Masereeuw R, Pickkers P (2013) Alkaline phosphatase as a treatment of sepsis-associated acute kidney injury. J Pharmacol Exp Ther 344:2–7
Beumer C, Wulferink M, Raaben W, Fiechter D, Brands R, Seinen W (2003) Calf intestinal alkaline phosphatase, a novel therapeutic drug for lipopolysaccharide (LPS)-mediated diseases, attenuates LPS toxicity in mice and piglets. J Pharmacol Exp Ther 307:737–744
Rosin DL, Hall JP, Zheng S, Huang L, Campos-Bilderback S, Sandoval R, Bree A, Beaumont K, Miller E, Larsen J, Hariri G, Kaila N, Encarnacion IM, Gale JD, van Elsas A, Molitoris BA, Okusa MD (2022) Human recombinant alkaline phosphatase (ilofotase alfa) protects against kidney ischemia-reperfusion injury in mice and rats through adenosine receptors. Front Med (Lausanne) 9:931293
Peters E, Ergin B, Kandil A, Gurel-Gurevin E, van Elsas A, Masereeuw R, Pickkers P, Ince C (2016) Effects of a human recombinant alkaline phosphatase on renal hemodynamics, oxygenation and inflammation in two models of acute kidney injury. Toxicol Appl Pharmacol 313:88–96
Davidson JA, Khailova L, Treece A, Robison J, Soranno DE, Jaggers J, Ing RJ, Lawson S, Lujan SO (2019) Alkaline phosphatase treatment of acute kidney injury in an infant piglet model of cardiopulmonary bypass with deep hypothermic circulatory arrest. Sci Rep 9:14175
Kiffer-Moreira T, Sheen CR, Gasque KC, Bolean M, Ciancaglini P, van Elsas A, Hoylaerts MF, Millán JL (2014) Catalytic signature of a heat-stable, chimeric human alkaline phosphatase with therapeutic potential. PLoS ONE 9:e89374
Heemskerk S, Masereeuw R, Moesker O, Bouw MP, van der Hoeven JG, Peters WH, Russel FG, Pickkers P (2009) Alkaline phosphatase treatment improves renal function in severe sepsis or septic shock patients. Crit Care Med 37(417–423):e411
Pickkers P, Mehta RL, Murray PT, Joannidis M, Molitoris BA, Kellum JA, Bachler M, Hoste EAJ, Hoiting O, Krell K, Ostermann M, Rozendaal W, Valkonen M, Brealey D, Beishuizen A, Meziani F, Murugan R, de Geus H, Payen D, van den Berg E, Arend J (2018) Effect of human recombinant alkaline phosphatase on 7-day creatinine clearance in patients with sepsis-associated acute kidney injury: a randomized clinical trial. JAMA 320:1998–2009
Pickkers P, Snellen F, Rogiers P, Bakker J, Jorens P, Meulenbelt J, Spapen H, Tulleken JE, Lins R, Ramael S, Bulitta M, van der Hoeven JG (2009) Clinical pharmacology of exogenously administered alkaline phosphatase. Eur J Clin Pharmacol 65:393–402
Levy MM, Evans LE, Rhodes A (2018) The surviving sepsis campaign bundle: 2018 update. Intensive Care Med 44:925–928
Naorungroj T, Neto AS, Wang A, Gallagher M, Bellomo R (2022) Renal outcomes according to renal replacement therapy modality and treatment protocol in the ATN and RENAL trials. Crit Care 26:269
Bagshaw SM, Neto AS, Smith O, Weir M, Qiu H, Du B, Wang AY, Gallagher M, Bellomo R, Wald R (2022) Impact of renal-replacement therapy strategies on outcomes for patients with chronic kidney disease: a secondary analysis of the STARRT-AKI trial. Intensive Care Med 48:1736–1750
Ronco C, Bellomo R, Kellum JA (2019) Acute kidney injury. Lancet 394:1949–1964
Joannidis M, Druml W, Forni LG, Groeneveld ABJ, Honore PM, Hoste E, Ostermann M, Oudemans-van Straaten HM, Schetz M (2017) Prevention of acute kidney injury and protection of renal function in the intensive care unit: update 2017: Expert opinion of the Working Group on Prevention, AKI section, European Society of Intensive Care Medicine. Intensive Care Med 43:730–749
Forni LG, Darmon M, Ostermann M, Oudemans-van Straaten HM, Pettilä V, Prowle JR, Schetz M, Joannidis M (2017) Renal recovery after acute kidney injury. Intensive Care Med 43:855–866
Liu C, Peng Z, Dong Y, Li Z, Song X, Liu X, Andrijasevic NM, Gajic O, Albright RC Jr, Kashani KB (2021) Continuous renal replacement therapy liberation and outcomes of critically ill patients with acute kidney injury. Mayo Clin Proc 96:2757–2767
Li Y, Li H, Zhang D (2019) Timing of continuous renal replacement therapy in patients with septic AKI: a systematic review and meta-analysis. Medicine (Baltimore) 98:e16800
Ostermann M, Chang R (2008) Correlation between the AKI classification and outcome. Crit Care 12:R144
Acknowledgements
PIs of all enrolling sites and part of the REVIVAL investigators are depicted in supplementary file 2. The authors would like to thank all participating patients, their families for their support, as well as study coordinators and research nurses of all participating sites in delivering the REVIVAL trial. The members of the data monitoring committee are thanked for their rigorous review of the data throughout the trial process. The authors would also like to thank Danielle Forkink (AM-Pharma, Clinical Trial Coordinator), Mariam Hamed (Phastar Programming Consultant), Annelies Legters (AM-Pharma, Sr. Director, Global Clinical Trials), and Sharon Richards (Phastar Biostatistician Consultant) for their support.
The REVIVAL investigators: Rinaldo Bellomo, Angus Carter, Dietmar Fries, Michael Joannidis, Philip Eller, Eric Hoste, Ludovic Gérard, Nicolas DeSchryver, Elisabeth Diltoer, Vincent Huberlant , Isabelle Michaux, Patrick M. Honore, Tom Fivez, Christopher Doig, Gordon Wood , John Boyd, Scott Millington, Alexis Turgeon, Maj Kamper, Thomas Strøm, Sussanne Iversen, Hendrik Gammelager, Bodil Steen Rasmussen, Christoffer Grant Sølling, Morten Hyllander Mæller, Thorbjoern Groefte , Nilanjan Dey, Ulf Gøttrup Pedersen, Mila Valkonen, Panu Uusalo, Ville Jalkanen, Ferhat Meziani, Jermie Lemarie, Gaetan Plantefeve, Konstantimos Bachoumas, Jean Louis Dufour, Anne-Laure Fedou, Pierre Asfar, Xavier Monnet, Christophe Vinsonneau, Sebastien Gibot, Christophe Guitton, Jean-Pierre Quenot, Gregoire Muller, Jean Yves Lefrant, Emmanuelle Mercier, Alexandre Mebazaa, Melanie Meersch, Andreas Kortgen, Sebastian Fichtner, Stefan Kluge, Gernot Marx, Alistair Nichol, Ignatio Martin-Loeches, Bairbre McNicolas, Hidenobu Kamohara, Masahiro Harada, Takuo Nakagami, Shingo Adachi, Kohei Ota , Ryo Furuya, Ayumu Tsuruoka, Yasuaki Mizushima, Satoki Inoue, Pieter Tuinman, F. Wim Roozendaal, Peter Pickkers, Bert Beishuizen, Oscar Hoiting, Tom Dormans, Arthur Van Zanten, Paul Young, Anthony Williams, Colin McArthur, Pawel Twardowski, Shay McGuinness, Ricard Ferrer Roca, Carol Lorencio Cardenas, Anna Navas Perez, Fernando Martinez Sagasti, Marlies Ostermann, Ingeborg Welters, Matt Wise, Sam Waddy, Niall MacCallum, Raghaven Murugan, Hernando Gomez, Larry Busse, David Boldt, Andrew Bernard, Daniel Files, Benjamin Margolis, Jarrod Mosier, Jonathon Truwit , Felix Zamora, Danielle Davison, Matthew Exline, Nathan Nielsen, Duncan Hite.
Funding
This work was supported by AM-Pharma. The role of the sponsor in the design of the study was to coordinate and facilitate processes, where the scientific input was provided by the members of the protocol committee, steering committee, and specific input by external experts in data management and statistics. The sponsor contracted an external contract research organization to operationally conduct the study at the study sites. The contract research organization was responsible for setting up the technical systems, data collection, quality control, pharmacovigilance, statistics, and further overall management of the study, under coordination and supervision of the sponsor. The statistical analysis plan was prepared by the contract research organization with input by principal investigator, sponsor, and external experts in statistics. The analyses were performed by external contract research organizations. Data were interpreted by the members of the steering committee, and, in a later phase, all coauthors and external experts, coordinated by the sponsor, could provide input. The principal investigator was responsible for preparation of the manuscript. All coauthors reviewed, made adjustments, and approved the manuscript. The decision to submit the manuscript was made by the principal investigator and other coauthors.
Author information
Authors and Affiliations
Consortia
Corresponding author
Ethics declarations
Conflicts of interest
PP has received travel reimbursements and consulting fees from AM-Pharma in relation to his role as PI for REVIVAL, and consulting fees from Adrenomed, EBI Paion, Sphingotec, and 4Teen4 outside the submitted work. DCA has received consulting fees from AM-Pharma. KB, AM-Pharma BV, The Netherlands. RB has received consulting fees and research support from AM-Pharma, Baxter, Paion, Viatris, Jafron Biomedical, and CSL Behring. EvdB, AM-Pharma BV, The Netherlands. JB, AM-Pharma BV, The Netherlands. MHB has received consulting fees from AM-Pharma in relation to his role for REVIVAL and has conducted contract research for Inotrem outside of the submitted work. KD has received consulting fees from AM-Pharma. CJD reports no conflicts of interest. RF has received consulting fees from AM-Pharma. BF has received consulting fees from AM-Pharma as a member of the REVIVAL steering committee, and consulting fees from Inotrem, Aridis, and Enlivex outside the submitted work. HG reports funding from various companies in the form of research grants to (and administered by) Aarhus University or Aarhus University Hospital. HG has received support for attending meetings by Baxter A/S. UGP reports no conflicts of interest. EH has received a travel grant from AM-Pharma. SI reports no conflicts of interest. Michael Joannidis has received honoraria or research support from Baxter Healthcare Corp, AM-Pharma, CLS Behring, Fresenius, Takeda, Sanofi and Novartis. JAK discloses fees paid by AM-Pharma in relation to his role as national PI for REVIVAL and is currently a full-time employee of Spectral Medical. KL has been a member of the REVIVAL Steering Committee for AM Pharma. She has been a consultant/member of the DSMB for Seastar, Novartis, BOA Medical, Baxter, and Biomerieux, and she holds stock in Amgen. MM has received lecture fees from Baxter and Fresenius Medical Care. RM reports honoraria for consulting from Baxter, Biomerieux, Mallinckrodt, GE Healthcare, Sanofi, Abiomed, NovaBiomed, Renasym, and advisory board reimbursements from AM Pharma, Renibus, Alexion, Novartis, and Guard. SM reports no conflicts of interest. PTM has received consulting fees from AM-Pharma (for Clinical Trial Steering Committee activities), Novartis, Renibus Therapeutics, and Alexion. AN reports an unrestricted grant from Baxter to support the renal substudy of the TAME trial. MO has received speaker honoraria from Fresenius Medical, Baxter and Biomerieux; her institution received research funding from Baxter, Fresenius Medical, Biomerieux, and LaJolla Pharma. CS reports no conflicts of interest. PV reports no conflicts of interest. MW, AM-Pharma BV, The Netherlands. PJY has received consulting fees from AM Pharma and from Baxter Healthcare Pty. AZ has received consulting fees from Astute-Biomerieux, Baxter, Bayer, Novartis, Guard Therapeutics, AM Pharma, Paion, Renibus, Fresenius, research funding from Astute-Biomerieux, Fresenius, Baxter, and speakers fees from Astute-Biomerieux, Fresenius, Baxter.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The REVIVAL Investigators are listed in the Acknowledgement section.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Pickkers, P., Angus, D.C., Bass, K. et al. Phase-3 trial of recombinant human alkaline phosphatase for patients with sepsis-associated acute kidney injury (REVIVAL). Intensive Care Med 50, 68–78 (2024). https://doi.org/10.1007/s00134-023-07271-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00134-023-07271-w