Abstract
Purpose
The incidence, patient features, risk factors and outcomes of surgery-associated postoperative acute kidney injury (PO-AKI) across different countries and health care systems is unclear.
Methods
We conducted an international prospective, observational, multi-center study in 30 countries in patients undergoing major surgery (> 2-h duration and postoperative intensive care unit (ICU) or high dependency unit admission). The primary endpoint was the occurrence of PO-AKI within 72 h of surgery defined by the Kidney Disease: Improving Global Outcomes (KDIGO) criteria. Secondary endpoints included PO-AKI severity and duration, use of renal replacement therapy (RRT), mortality, and ICU and hospital length of stay.
Results
We studied 10,568 patients and 1945 (18.4%) developed PO-AKI (1236 (63.5%) KDIGO stage 1500 (25.7%) KDIGO stage 2209 (10.7%) KDIGO stage 3). In 33.8% PO-AKI was persistent, and 170/1945 (8.7%) of patients with PO-AKI received RRT in the ICU. Patients with PO-AKI had greater ICU (6.3% vs. 0.7%) and hospital (8.6% vs. 1.4%) mortality, and longer ICU (median 2 (Q1-Q3, 1–3) days vs. 3 (Q1-Q3, 1–6) days) and hospital length of stay (median 14 (Q1-Q3, 9–24) days vs. 10 (Q1-Q3, 7–17) days). Risk factors for PO-AKI included older age, comorbidities (hypertension, diabetes, chronic kidney disease), type, duration and urgency of surgery as well as intraoperative vasopressors, and aminoglycosides administration.
Conclusion
In a comprehensive multinational study, approximately one in five patients develop PO-AKI after major surgery. Increasing severity of PO-AKI is associated with a progressive increase in adverse outcomes. Our findings indicate that PO-AKI represents a significant burden for health care worldwide.
Similar content being viewed by others
One in five patients develop postoperative acute kidney injury (PO-AKI) after major surgery and adverse outcomes progressively increase with increasing severity of PO-AKI. These findings indicate that postoperative AKI represents a significant burden for health care worldwide |
Introduction
Over 300 million patients undergo major surgery each year worldwide with the potential to cause acute kidney injury (AKI) [1]. Nevertheless, the exact incidence of surgery associated, postoperative AKI (PO-AKI) remains unknown [2]. Retrospective data suggest that 1.8–39.3% of the patients develop PO-AKI after abdominal [3,4,5,6,7] and 3.1–39.9% after cardiac surgical procedures [8, 9]. One major drawback of these retrospective studies is that they often lack data on urine output. This criterion is important for the correct diagnosis of AKI [10, 11]. Without urine output, the incidence of PO-AKI will be underestimated. Also, the presence of both Kidney Disease: Improving Global Outcomes (KDIGO) criteria for AKI definition, reduction of urine output and increase of creatinine, predicts worse outcomes compared to AKI based on the creatinine criterion alone [10]. In addition, AKI rates may further vary between different patient populations across different countries. The type, duration, and technique of surgery, as well as the AKI definition used are factors that influence PO-AKI rates. Moreover, the inclusion or non-inclusion of patients from low or middle-income countries play an important role. The lack of knowledge in terms of PO-AKI incidence is a problem as it may lead to underestimates of the risk of PO-AKI in this field, the burden it poses on a global scale and fail to identify risk factors for its development.
Therefore, the aim of this study was to prospectively investigate the incidence of PO-AKI within 72 h after major cardiac and non-cardiac surgery and to evaluate pre- and intraoperative risk factors for PO-AKI in an international study with strong representation of middle and low-income countries [12].
Methods
Study design and ethics
The epidemiology of surgery associated acute kidney injury (EPIS-AKI) study is an international prospective, observational, multicenter, cohort study. The design of the trial has been published previously [12]. Participating centers were approached by the different national societies (partly by direct inquiry, partly by presenting the study at national congresses and/or newsletters) supporting this trial (see Acknowledgements). Center participation was voluntary. Primary approval was obtained from the Research Ethics Committee of the Chamber of Physicians Westfalen-Lippe and the Westphalian Wilhelms-University Münster (2019-424-f-S). Country-specific requirements, including local ethics approval and/or study registration were fulfilled according to the local requirements and prior to patient enrollment. The trial was registered prior to initiation of the study at clinicaltrials.gov (NCT04165369, November 18th 2019). The manuscript follows the principles of “Strengthening the Reporting of Observational Studies in Epidemiology” (STROBE) and the Declaration of Helsinki (Fortaleza 2013).
Patient recruitment and consenting
All patients (age ≥ 18 years) undergoing major surgery (≥ 2 h) with the need of subsequent intensive care unit (ICU) or high dependency unit (intermediate care or post anesthesia care unit) admission were consecutively included in this study. All surgical specialties were considered (including emergencies). Exclusion criteria were: pre-existing AKI, AKI within the last 3 months, end-stage renal disease with dialysis dependency, kidney transplant. No patient was excluded from the study based on sex, ethnicity or religion. Written informed consent was obtained from patients whenever requested by local authorities before inclusion following local regulations.
Data collection
Data were collected in a protected web platform (Research Electronic Data Capture, V.10.6.22, Vanderbilt University) and confidentially stored in a deidentified form on secured servers at the University of Münster.
Diagnosis and severity of PO-AKI were assessed by serum-creatinine and/or urine output according to the KDIGO criteria [13]. The latest serum creatinine before surgery was defined as baseline value. Serum creatinine was measured at least once a day for the first 3 days. Patients with serum creatinine increases of ≥ 0.3 mg/dl within 48 h or 1.5–1.9 times increases within 72 h after surgery were diagnosed KDIGO stage 1, patients with serum creatinine increases of 2–2.9 times within 72 h were diagnosed KDIGO stage 2, and patients with serum creatinine increases of ≥ 3 times or ≥ 4 mg/dl or with the need of renal replacement therapy (RRT) were diagnosed KDIGO stage 3. Further, patients with a urine output < 0.5 ml/kg/h for ≥ 6 h were diagnosed KDIGO stage 1, urine output < 0.5 ml/kg/h for ≥ 12 h KDIGO stage 2, and < 0.3 ml/kg/h for ≥ 24 h or anuria for ≥ 12 h KDIGO stage 3. Once the urine catheter was removed, the urine output criterion could no longer be considered. Data collection included PO-AKI diagnosis and severity.
After 90 days, a follow-up was performed by telephone by contacting either the patient or the general practitioner in order to ask for survival, need for RRT and latest creatinine value.
For country comparisons, we used different world zone classifications according to their latest publication: the United Nations (UN) geoscheme [14], and country’s health expenditure as percentage of gross domestic product 2019 as reported by the World Health Organization [15].
Outcomes
The primary outcome was PO-AKI within the first 72 h after surgery. Secondary outcomes were severity of PO-AKI, duration of PO-AKI (transient < 48 h vs. persistent ≥ 48 h) [16], use of RRT including the type of RRT (continuous RRT (CRRT), prolonged intermittent RRT (PIRRT) intermittent hemodialysis (IHD)), ICU and hospital mortality, length of ICU and hospital stay as well as the occurrence of major adverse kidney events at day 90 (MAKE90) which is a combined endpoint consisting of mortality, need for RRT and persistent renal dysfunction (defined as serum-creatinine ≥ 1.5 times as compared to baseline serum-creatinine). Furthermore, analyses were performed for the effect of pre-/perioperative risk factors on the incidence of PO-AKI.
Sample size calculation
The primary aim of the study was to estimate the rates of PO-AKI and to derive the corresponding exact two-sided 95% confidence interval (CI) according to Clopper-Pearson. Depending on the type of surgery, PO-AKI rates between 1.8 and 39.3% were reported in the literature [3,4,5,6]. Therefore, a rate of 40% was assumed for the sample size calculation as a conservative approach. Using this assumption, 10,000 patients are needed to limit the width of the 95% CI to 1.9%. Thus, with n = 10,000 patients, the incidence of PO-AKI can be estimated with at least this precision.
Statistical analysis
Statistical analyses were planned prior to reviewing the data. Frequencies, percentages, medians, quartiles and p-values were calculated for the baseline variables and secondary endpoints as applicable.
Fisher's exact test and Pearson’s Chi-squared test were used to compare categorical variables between groups. Continuous variables were compared using Welch's t-test or Wilcoxon signed-rank test depending on whether the target variable was normally distributed in both groups or not. All secondary endpoints were censored at day 90.
CIs for binomial proportion estimates, e.g., the development of the primary endpoint PO-AKI within 72 h after surgery, were calculated using the Clopper-Pearson exact method with a 95% confidence level. For multinomial proportion estimates, e.g. KDIGO stages (1/2/3) in PO-AKI patients, simultaneous 95% CIs were calculated using Goodman’s methods [17].
To identify and assess the association of further risk factors for PO-AKI, multivariable logistic regression analyses were performed. In a first step, we selected variables that, according to previous clinical knowledge, could be associated with PO-AKI. The following variables were selected in this way: sex, age, body mass index (BMI), UN-geoscheme, health expenditure, hypertension, atrial fibrillation, myocardial infarction, congestive heart failure, diabetes, chronic obstructive pulmonary disease (COPD), chronic kidney disease (CKD), peripheral vascular disease, stroke, American Society of Anesthesiologists (ASA) score, urgency of procedure, surgery duration, type of surgery, use of cell saver, transfusion, fluid balance, blood loss, hypotensive episodes, intraoperative complications, use of nephrotoxic agents, use of vasopressors. We included all these variables in a logistic regression model and then performed a fast backward variable selection based on Akaike’s Information Criterion (AIC) to identify a reasonable set of potential risk factors for PO-AKI. In each iteration, the influencing variable whose exclusion caused the greatest reduction of the AIC compared to the current model was excluded from the current model until no omission of a single variable resulted in a further reduction of the AIC.
To further investigate the risk of different intraoperative vasopressors for PO-AKI, we restricted the data to patients who received vasopressors and formed two groups: vasopressors (received norepinephrine and/or vasopressin and/or other vasopressors) and inotropes (received epinephrine and/or dobutamine). On this data subset, we fitted a new logistic regression model including combinations of vasopressors as well as all other risk factors identified by variable selection performed in the main PO-AKI analysis.
In a final step, we repeated the variable selection and model fitting procedure on the PO-AKI subgroup to identify potential risk factors for the duration of PO-AKI (persistent vs. transient).
All p-values and confidence limits were two-sided. Only the confidence interval of the primary endpoint is to be interpreted confirmatory. The secondary endpoints were not adjusted for multiple testing, p-values of secondary statistical analyses are therefore regarded statistically noticeable (significant) in case p ≤ 0.05. An overall significance level across all secondary statistical analyses was not determined and cannot be calculated. In all analyses, only the complete cases were considered, i.e., missing values were not imputed. Statistical analyses were conducted using R (Version R-4.1.2).
Results
Participants
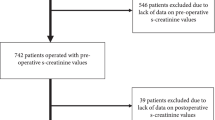
From June 2020 to December 2021, we included 10,568 patients from 30 countries and 148 centers (Fig. 1, Supplementary eFigure 1). The median age was 62 (Q1, Q3, 52, 71) years. 6456 (59.7%) were male and 7643 Caucasian (72.3%), most surgeries were elective procedures (96.1%). Baseline characteristics are demonstrated in Table 1, surgical details in Supplementary eTable 1. There were large regional differences in included types of surgeries (Supplementary eTable 2 and eTable 3).
Postoperative acute kidney injury
Overall, 1945 of 10,568 (18.4% [95% CI 17.7–19.2%]) patients developed PO-AKI within 72 h after surgery (primary endpoint; KDIGO stage 1: 1236 (63.5% [95% CI 60.8–66.2%]), KDIGO stage 2: 500 (25.7% [95% CI 23.4–28.2%]), KDIGO stage 3: 209 (10.7% [95% CI 9.1–12.6%]) (Fig. 2).
In 33.8% [95% CI 31.7–35.9%] PO-AKI was persistent and in 1482 (76.2%) PO-AKI occurred within the first 24 h after surgery (15.8% at day 2 and 7.9% at day 3). Of PO-AKI cases, 856 (44% [95% CI 41.3–46.8%]) were diagnosed by serum-creatinine, 614 (31.6% [95% CI 29–34.2%]) by urine output and 475 (24.4% [95% CI 22.1–26.9%]) by both criteria (Fig. 2). PO-AKI was most frequent in patients undergoing urologic (162/586 (27.6%)), cardiac (802/3101 (25.9%)), vascular (132/532 (24.8%)) and general surgery 571/3170 (18%)) (Table 1).
Intra- and postoperatively, patients with PO-AKI received significantly more fluids, had significantly higher blood loss, and received significantly more vasopressors (Supplementary eTable 1). Additionally, the proportion of patients who were administered nephrotoxic agents in the postoperative phase was significantly higher compared to patients without PO-AKI (Supplementary eTable 1).
Regional differences
There were significant differences in the rates of PO-AKI according to region. North America had the highest PO-AKI rate (47.3%), followed by Europe (22.7%), Asia (13.8%), South America (13%), and Africa (8.5%) (Supplementary eTable 4, Fig. 3a).
According to percentage of health expenditure, countries with > 10% showed the highest rates (31.1%), followed by less than 5% (14.3%) and 5–10% (13%) (Fig. 3b, Supplementary eTable 4). However, the rate of persistent PO-AKI was similar across regions with nearly one third of the patients developing persistent PO-AKI. Across all regions, in-hospital mortality was significantly higher in PO-AKI patients as compared to non-PO-AKI patients. However, within the subgroup of PO-AKI, mortality rates differed across regions with Africa showing the highest mortality rates (23.9%) compared to Asia (9.8%), Europe (7.2%), and North America (4.5%).
Secondary endpoints
In total, 164 (8.4% [95% CI 7.2–9.8%]) PO-AKI patients were treated with RRT during their ICU stay (73.8% CRRT, 18.9% IHD, and 7.3% PIRRT) and 184 (9.5% [95% CI 8.2–10.8%]) PO-AKI patients during hospital stay (Table 2). Patients with PO-AKI had significantly longer ICU (median 3 (Q1, Q3, 1, 6) days vs. 2 (Q1, Q3, 1, 3) days) as well as hospital length of stays (median 14 (Q1, Q3, 9, 24) days vs. 10 (Q1, Q3, 7, 17) days), higher mortality rates in the ICU (6.3% [95% CI 5.2–7.4%] vs. 0.7% [95% CI 0.5–0.9%]) and in hospital (8.5% [95% CI 7.3–9.8% vs. 1.3% [95% CI 1.1–1.6%]) compared to non-PO-AKI patients (Table 2) (p < 0.001). Endpoint rates increased with increasing severity of PO-AKI (Table 2) and mortality rates were highest in KDIGO3 patients meeting both criteria of the AKI definition (Fig. 4).
Patients with PO-AKI showed significantly higher rates of MAKE90 as compared to patients without PO-AKI (21.1% vs. 8.5%; p < 0.001) (Table 2). Rates increased significantly with the severity of PO-AKI (KDIGO 1, 14.9%; KDIGO 2, 23.1%; KDIGO 3, 53.1%; p < 0.001).
Risk factor assessment for PO-AKI
A multivariable regression analysis with backward variable selection showed that male sex, increased age, a high percentage of health expenditure, hypertension, atrial fibrillation, congestive heart failure, diabetes, COPD, CKD, high ASA score, emergency procedures, long surgery duration, certain types of surgery, cell saver, transfusion, positive fluid balance, intraoperative complications such as bleeding and pulmonary complications, aminoglycosides and intraoperative use of vasopressors were risk factors for PO-AKI; whereas the region according to UN-geoscheme was not (Table 3). When investigating the effects of different vasopressors on the risk of PO-AKI among those patients receiving vasopressors, none of the combinations showed an increased risk (Supplementary eTable 5). Pre-existing CKD was a key risk factors for persistent PO-AKI (Supplementary eTable 6).
A multivariable regression analysis for hospital mortality showed PO-AKI as a key risk factor (OR, 5.40 [95% CI 4.10, 7.13]; p < 0.001) (Supplementary eTable 7).
Discussion
The EPIS-AKI trial is the first prospective international observational trial that focused exclusively on PO-AKI using the full KDIGO classification across multiple geographic settings and country-based income levels. Thus, it provides important data on the occurrence rate, morbidity, and mortality of PO-AKI. EPIS-AKI shows that PO-AKI is globally common in the perioperative period affecting approximately one in five patients, with urologic, cardiac and vascular surgery patients being most commonly affected and with one third of the patients developing persistent PO-AKI. Regional comparisons showed that Africa had the lowest incidence of PO-AKI but the highest mortality rates. Moreover, EPIS-AKI found that the intraoperative use of vasopressors was a risk factor for PO-AKI as was the use of aminoglycosides. Finally, mortality and ICU and hospital length of stay were significantly higher in patients with PO-AKI.
In non-surgical ICU patients, nearly every second patient develops AKI [18] but for the surgical setting, exact PO-AKI rates are unknown. Prior studies have demonstrated large variations in rates of PO-AKI, ranging from 1.8% to 39.3% in major abdominal surgery [6, 19], and from 3.1% to 39.9% in cardiac surgery [9, 20]. These differences appear due to variations in definitions and type of surgery (e.g. some studies only including hepatobiliary surgery, others including all types of major abdominal surgery). The EPIS-AKI study included all types of major surgery, cardiac as well as non-cardiac surgeries, and included patients admitted to high-dependency units, not just ICU. The overall rate of PO-AKI was 18.4% when using both criteria of the KDIGO definition (serum-creatinine and urine output).
In terms of the diagnostic criteria of the KDIGO definition, the urine output criterion is difficult to assess in daily clinical routine as an exact assessment is best in patients with an indwelling urinary catheter. However, our data shows the importance of the urine output criterion, which is highly associated with adverse outcomes in patients with AKI. Thus, the urine output criterion deserves special attention and should not be underestimated.
In terms of duration of PO-AKI, no other studies exclusive to surgery patients have focused on persistent PO-AKI. However, the finding that patients meeting both criteria of the KDIGO definition show worse outcomes as compared to those meeting only the urine output criterion, is comparable to other clinical settings [10].
Regional comparisons showed that there were significant differences in the rates of PO-AKI with countries with a high expenditure on health care having the highest PO-AKI rates. This may derive from access to comprehensive resources like extensive monitoring methods (i.e., multiple laboratory testing per day, electronic patient file, etc.) but also from the fact that there were large regional differences in the type of surgeries included as well as the selection of higher risk patients. The high differences in mortality rates cannot be explained by our data but may be a result from lack of interventions and unmeasured confounders such as frailty. Additionally, there is an increasing proportion of elderly patients with multiple long-term conditions undergoing surgery in high developed countries [21], who are particularly at risk for PO-AKI. In contrast to the higher PO-AKI rate, PO-AKI-associated mortality was inversely related to the percentage of the gross domestic product spent on health expenditure. This dissociation between incidence and outcome has been observed in other studies [22, 23]. It also suggests that less severe PO-AKI may be missed in lower resourced regions with higher severity cases driving increased mortality.
At first glance, mortality appears to be high at 3% for the total cohort. However, median Acute Physiology and Chronic Health Evaluation (APACHE) score and Simplified Acute Physiology Score (SAPS) were 8 (Q1, Q3, 5–13) and 20 (Q1, Q3, 12–29), respectively, which is within the normal estimated range (APACHE 5–9) [24]. Estimated mortality rates are approximately 3% for post-OP patients with SAPS of 20 points [25].
Prevention is key in the setting of PO-AKI. However, the pathophysiology of AKI, especially in the perioperative setting, is very complex. A large number of risk factors are not modifiable such as older age or comorbidities. However, some risk factors may be modifiable. For example, in our multivariable analysis, the administration of nephrotoxic agents, especially aminoglycosides, bleeding complications and the treatment of these with transfusion as well as fluid management were identified as risk factors for AKI. Alternative drugs to nephrotoxins exist and the use of nephrotoxic drugs should be carefully considered and avoided whenever possible. In this regard, a nephrotoxic stewardship approach to oversee the use of these substances has been proposed [26]. Transfusion as well as hypervolemia are known factors associated with AKI and a critical appraisal of their use is also important [27, 28]. Surgical factors such as duration and urgency of the procedure are factors that may be modified by identifying patients who are at risk for AKI, by informing the surgical team, and by prioritizing these patients. While intraoperative hypotension was not a risk factor for AKI, vasopressors were. This finding cannot be further explained with our data. However, it is conceivable that vasopressors represent a surrogate marker for less stable hemodynamics. On the other hand, there are also data showing that higher vasopressor use affects renal function due to decreased renal perfusion [29, 30]. Ultimately, this finding needs to be further investigated in additional studies.
The strengths of the EPIS-AKI trial are the large cohort of patients, the international setting with various participating centers and regions that have scarcely been described so far (e.g. Africa), the multiple types of surgery, the use of the full KDIGO criteria as well as detailed data on vasopressors, nephrotoxins and fluids.
However, the study has also several limitations. First, center participation was voluntary. Therefore, it remains uncertain whether the study cohorts are representative of other centers in the same country as well as non-participating countries in the same region. In addition, the number of included patients was very low in some countries and regions. Most centers originated from countries that spent ≥ 5% of gross domestic product (GDP) on total health expenditure. However, EPIS-AKI is one of the few studies that included a substantial number of countries, which spent less than 5% GDP on health. Second, some specialties or procedures are underrepresented creating a degree of selection bias. Third, we cannot exclude a certain degree of measurement bias for the following reasons. In some centers or patient’s creatinine may have been measured more frequently than in others. Moreover, it was not possible to consider the urine output criterion in every patient up to 3 days because of early urinary catheter removal. However, in a pragmatic study like ours, this bias cannot be controlled for as this represents daily clinical routine. Fourth, although aminoglycosides were found to be modifiable risk factors for PO-AKI, our data does not allow to perform dose-dependency analyses.
Conclusion
In a comprehensive multinational study, approximately one in five patients develop PO-AKI after major surgery. Increasing severity of PO-AKI is associated with a progressive increase in adverse outcomes. Our findings indicate that PO-AKI represents a significant burden for health care worldwide.
References
Weiser TG, Haynes AB, Molina G, Lipsitz SR, Esquivel MM, Uribe-Leitz T, Fu R, Azad T, Chao TE, Berry WR, Gawande AA (2015) Estimate of the global volume of surgery in 2012: an assessment supporting improved health outcomes. Lancet 385(Suppl 2):S11
Prowle JR, Forni LG, Bell M, Chew MS, Edwards M, Grams ME, Grocott MPW, Liu KD, McIlroy D, Murray PT, Ostermann M, Zarbock A, Bagshaw SM, Bartz R, Bell S, Bihorac A, Gan TJ, Hobson CE, Joannidis M, Koyner JL, Levett DZH, Mehta RL, Miller TE, Mythen MG, Nadim MK, Pearse RM, Rimmele T, Ronco C, Shaw AD, Kellum JA (2021) postoperative acute kidney injury in adult non-cardiac surgery: joint consensus report of the acute disease quality initiative and perioperative quality initiative. Nat Rev Nephrol. https://doi.org/10.1038/s41581-021-00418-2
Murugan R, Kellum JA (2011) Acute kidney injury: what’s the prognosis? Nat Rev Nephrol 7:209–217
Sun LY, Wijeysundera DN, Tait GA, Beattie WS (2015) Association of intraoperative hypotension with acute kidney injury after elective noncardiac surgery. Anesthesiology 123:515–523
Grams ME, Sang Y, Coresh J, Ballew S, Matsushita K, Molnar MZ, Szabo Z, Kalantar-Zadeh K, Kovesdy CP (2016) Acute kidney injury after major surgery: a retrospective analysis of veterans health administration data. Am J Kidney Dis 67:872–880
Bihorac A, Yavas S, Subbiah S, Hobson CE, Schold JD, Gabrielli A, Layon AJ, Segal MS (2009) Long-term risk of mortality and acute kidney injury during hospitalization after major surgery. Ann Surg 249:851–858
Myles PS, Bellomo R, Corcoran T, Forbes A, Peyton P, Story D, Christophi C, Leslie K, McGuinness S, Parke R, Serpell J, Chan MTV, Painter T, McCluskey S, Minto G, Wallace S, Australian, New Zealand College of Anaesthetists Clinical Trials N, the A, New Zealand Intensive Care Society Clinical Trials G (2018) Restrictive versus liberal fluid therapy for major abdominal surgery. N Engl J Med 378:2263–2274
Brunelli SM, Waikar SS, Bateman BT, Chang TI, Lii J, Garg AX, Winkelmayer WC, Choudhry NK (2012) Preoperative statin use and postoperative acute kidney injury. Am J Med 125(1195–1204):e1193
Kim JY, Joung KW, Kim KM, Kim MJ, Kim JB, Jung SH, Lee EH, Choi IC (2015) Relationship between a perioperative intravenous fluid administration strategy and acute kidney injury following off-pump coronary artery bypass surgery: an observational study. Crit Care 19:350
Kellum JA, Sileanu FE, Murugan R, Lucko N, Shaw AD, Clermont G (2015) Classifying AKI by urine output versus serum creatinine level. J Am Soc Nephrol 26:2231–2238
Priyanka P, Zarbock A, Izawa J, Gleason TG, Renfurm RW, Kellum JA (2020) The impact of acute kidney injury by serum creatinine or urine output criteria on major adverse kidney events in cardiac surgery patients. J Thorac Cardiovasc Surg S0022–5223:30025–30029
Weiss R, Saadat-Gilani K, Kerschke L, Wempe C, Meersch M, Zarbock A, Investigators E-A (2021) EPIdemiology of surgery-associated acute kidney injury (EPIS-AKI): study protocol for a multicentre, observational trial. BMJOpen 11:e055705
Kellum JALN, Aspelin P, Barsoum RS, Burdmann EA, Goldstein SL, Herzog CA, Joannidis M, Kribben A, MacLeod AM, Mehta RL, Murray PT, Naicker S, Opal SM, Schaefer F, Schetz M, Uchino S (2012) KDIGO Clinical Practice Guideline for Acte Kidney Injury 2012. Kidney Int Suppl 2:1–138
Division. UNS (2022) Geographic regions.
Organization WH (2022) Global health expenditure database.
Chawla LS, Bellomo R, Bihorac A, Goldstein SL, Siew ED, Bagshaw SM, Bittleman D, Cruz D, Endre Z, Fitzgerald RL, Forni L, Kane-Gill SL, Hoste E, Koyner J, Liu KD, Macedo E, Mehta R, Murray P, Nadim M, Ostermann M, Palevsky PM, Pannu N, Rosner M, Wald R, Zarbock A, Ronco C, Kellum JA, Acute Disease Quality Initiative W (2017) Acute kidney disease and renal recovery: consensus report of the Acute Disease Quality Initiative (ADQI) 16 Workgroup. Nat Rev Nephrol 13:241–257
Goodman LA (1965) On simultaneous confidence intervals for multinomial proportions. Technometrics 7:247–254
Hoste EA, Bagshaw SM, Bellomo R, Cely CM, Colman R, Cruz DN, Edipidis K, Forni LG, Gomersall CD, Govil D, Honore PM, Joannes-Boyau O, Joannidis M, Korhonen AM, Lavrentieva A, Mehta RL, Palevsky P, Roessler E, Ronco C, Uchino S, Vazquez JA, Vidal Andrade E, Webb S, Kellum JA (2015) Epidemiology of acute kidney injury in critically ill patients: the multinational AKI-EPI study. Intensive Care Med 41:1411–1423
Cho E, Kim SC, Kim MG, Jo SK, Cho WY, Kim HK (2014) The incidence and risk factors of acute kidney injury after hepatobiliary surgery: a prospective observational study. BMCNephrol 15:169
Xu JR, Zhu JM, Jiang J, Ding XQ, Fang Y, Shen B, Liu ZH, Zou JZ, Liu L, Wang CS, Ronco C, Liu H, Teng J (2015) Risk factors for long-term mortality and progressive chronic kidney disease associated with acute kidney injury after cardiac surgery. Medicine 94:e2025
Fowler AJ, Abbott TEF, Prowle J, Pearse RM (2019) Age of patients undergoing surgery. Br J Surg 106:1012–1018
Susantitaphong P, Cruz DN, Cerda J, Abulfaraj M, Alqahtani F, Koulouridis I, Jaber BL, Acute Kidney Injury Advisory Group of the American Society of N (2013) World incidence of AKI: a meta-analysis. Clin J Am Soc Nephrol 8:1482–1493
Melo FAF, Macedo E, Fonseca Bezerra AC, Melo WAL, Mehta RL, Burdmann EA, Zanetta DMT (2020) A systematic review and meta-analysis of acute kidney injury in the intensive care units of developed and developing countries. PloSOne 15:e0226325
Knaus WA, Draper EA, Wagner DP, Zimmerman JE (1985) APACHE II: a severity of disease classification system. Crit Care Med 13:818–829
Le Gall JR, Lemeshow S, Saulnier F (1993) A new Simplified Acute Physiology Score (SAPS II) based on a European/North American multicenter study. JAMA 270:2957–2963
Gray MP, Barreto EF, Schreier DJ, Kellum JA, Suh K, Kashani KB, Rule AD, Kane-Gill SL (2022) Consensus obtained for the nephrotoxic potential of 167 drugs in adult critically ill patients using a modified Delphi method. Drug Saf 45:389–398
Rasmussen SR, Kandler K, Nielsen RV, Jakobsen PC, Ranucci M, Ravn HB (2020) Association between transfusion of blood products and acute kidney injury following cardiac surgery. Acta Anaesthesiol Scand 64:1397–1404
Garzotto F, Ostermann M, Martín-Langerwerf D, Sánchez-Sánchez M, Teng J, Robert R, Marinho A, Herrera-Gutierrez ME, Mao HJ, Benavente D, Kipnis E, Lorenzin A, Marcelli D, Tetta C, Ronco C (2016) The dose response multicentre investigation on fluid assessment (DoReMIFA) in critically ill patients. Crit Care 20:196
Bellomo R, Giantomasso DD (2001) Noradrenaline and the kidney: friends or foes? Crit Care 5:294–298
Chiu C, Fong N, Lazzareschi D, Mavrothalassitis O, Kothari R, Chen LL, Pirracchio R, Kheterpal S, Domino KB, Mathis M, Legrand M (2022) Fluids, vasopressors, and acute kidney injury after major abdominal surgery between 2015 and 2019: a multicentre retrospective analysis. Br J Anaesth 129:317–326
Acknowledgements
The members of the EPIS AKI investigators are: Algeria: Constantine Centre Hospitalier Universitaire (Hichem Makhloufi), Etablissement Hospitalier Spécialisé Salim Zemirli El Harrach (Rachida Sakhraoui), Saadna Abdenour Teaching Hospital (Amel Ouyahia, Mounira Rais, Aya Tinhinane Kouicem), Sabratha Libya National Cancer Institute (Khawla Derwish), University Hospital of Sétif (Meriem Abdoun, Ilhem Ouahab, Souad Bouaoud), University Hospital 1st November 1954 (Anisse Tidjane). Colombia: Universidad El Bosque, Fundación Cardioinfantil (Carlos Jose Pérez Rivera, Juan Pablo García). China: First Affiliated Hospital of Soochow University (Ke Peng, Fu-hai Ji, Zheng-min Ma). Czech Republic: University Hospital Ostrava, Department of Anesthesia and Intensive Care (Peter Sklienka), Egypt: Tanta University Faculty of Medicine (Mohamed Gamal Elbahnasawy, Shady Elsalhawy), Alexandria University Main Hospital (Ahmed Mahmoud Nafea), Alexandria University (Nermin A. Osman), Mansoura University, Faculty of Medicine, Gastrointestinal Surgical Center (Moataz Maher Emara, Mohamed Mamdouh Bonna), Zagazig University Hospital (Ibrahim Abdelmonaem Abdehaleem), Assiut University Hospital (Ahmed Mohamed Abbas, Mostafa Samy Abbas, Hany Mostafa Esmaeil). France: Centre Hospitalier Universitaire de Bordeaux (Oliver Joannes-Boyau), Centre Hospitalier Universitaire de Reims (Vincent Legros, Thierry Floch, Salvatore Muccio, Lison Menage-Innocenti, Benjamin Brochet, Marion Leclercq-Rouget), Centre Hospitalier Universitaire de Orléans (Claire Geneve, Bernardita Valenzuela Mocarquer), Hôpital Privé Sévigné (Christophe Aveline, Pierre Vautier), Hôpital Robert Schuman-Groupe UNEOS (Julien Nadaud), Hôpital Edouard Herriot (Thomas Rimmelé, Valérie Cerro), Gustave Roussy (Stéphanie Suria, Jamie Elmawieh , Rita El-Jawiche), Centre Hospitalier Universitaire de Lille (Cédric Cirenei, Gilles Lebuffe), Polyclinique de Limoges, Clinique François Chénieux (Sébastien Ponsonnard), Centre Hospitalier des Pays de Morlaix (Pierre-Yves Egreteau), Centre Hospitalier Universitaire de Nice, Université Côte d’Azur (Carole Ichai), Centre Hospitalier Dron Tourcoing (Vanessa Jean-Michel), Centre Hospitalier Universitaire d'Angers (Maxime Léger, Sigismond Lasocki, Charline Masson, Emmanuel Rineau , Viviane Cassisa), Centre François Baclesse (Pierre Verrier), Hôpital Bichat (Enora Atchade), Centre Hospitalier Simone Veil de Blois (Charles-Edouard Rochon), Centre Hospitalier de Bigorre (Vidal Quentin), Centre Hospitalier Universitaire de Bordeaux, Site Pellegrin (Nina Queixalos), Clinique Sainte Anne (Thierry Braun), Centre Hospitalier Libourne (Hubert Grand), Clinique Pasteur (Nicolas Mayeur, Marie Pasquie), Centre Hospitalier de Marne-La-Vallée (Pierre Garçon), Centre Hospitalier Universitaire de Nice (Vincent Bruckert), Centre Hospitalier Henri Mondor d'Aurillac (Gaël Pradel), Centre Hospitalier Mont de Marsan (Andersen Ramorasata), Centre Hospitalier de Dax (Céline Ravry), Clinique de la Sauvegarde (Nicolas Mottard). Germany: University Hospital Münster (Alexander Zarbock, Melanie Meersch, Raphael Weiss, Thilo von Groote, Christian Dörr, Mira Küllmar, Christina Massoth, Arash Motekallemi, Khaschayar Saadat-Gilani, Felix Albert, Laura Kerschke, Michael Storck, Julian Varghese, Carola Wempe), University Hospital RWTH Aachen (Linda Grüßer, Ana Kowark), University Hospital Düsseldorf (Timo Brandenburger), Kliniken Maria Hilf Mönchengladbach (Andreas Hohn), University Hospital Tübingen (Peter Rosenberger, Helene Häberle, Pascal Hofmann, Jonathan Kuhle, Stefanie Calov, Alice Marie Bernard, Valbona Mirakaj, Kathrin Weber, Kathrin Pfister, Lena Stetz), University Hospital Leipzig (Sarah Dorothea Müller), Herz-Jesu-Hospital Münster (Stephan Klaus, Marco Sadlo), St. Josef-Stift Sendenhorst (Christian Sengelhoff), Sankt Franziskus Hospital (Carina-Kristin Stenger, Ulrich Göbel) Hosspital Karlsburg (Matthias Heringlake). Greece: University Hospital Larissa (Eleni Arnaoutoglou), Laiko General Hospital of Athens (Panagiota Stratigopoulou), University Hospital Ioannina (Pantazi Danai), G. Gennimatas’ General Hospital of Athens (Antonia Dimakopoulou), General Hospital of Thessaloniki "George Papanikolaou" (Apostolos-Alkiviadis Menis), Aristotle University of Thessaloniki, General Hospital “George Papanikolaou” (Orestis Ioannidis). Iraq: Al-Diwaniyah Hospital (Humam Jalaawiy), Bagdad Medical City Hospital (Aeshah Anwar), Al-Nassiryah Teaching Hospital (Hashim Talib Hashim), Khanaqeen General Hospital (Hogir Imad Rasheed Aldawoody). Italy: Policlinico Paolo Giaccone (Andrea Cortegiani, Mariachiara Ippolito, Claudia Marino, Gabriele Presti, Dario Calogero Fricano), San Bortolo Hospital (Silvia De Rosa), Montebelluna City Hospital (Andrea Bianchin), San Carlo Regional Hospital (Gianluca Paternoster, Umberto Fasciano), Fondazione Policlinico Universitario A. Gemelli IRCCS and Catholic University of the Sacred Heart (Salvatore Lucio Cutuli), University of Ferrara ( Savino Spadaro, Enrico Bussolati , Marco Palmieri, Carlo Alberto Volta), Great Metropolitan Hospital "Bianchi-Melacrino-Morelli" (Vincenzo Francesco Tripodi), Sant'Eugenio Hospital (Diego Fiume), Frangipane Hospital (Angela Iuorio), General Hospital Vallecamonica (Clemente Santorsola). Jordan: Al-Basheer Hospital Amman (Bilal Abu-Hussein, Khaled Hasanein). (South) Korea: Yonsei University College of Medicine (Seokyung Shin), University Hospital Yeungnam, Yeungnam School of Medicine (Jongyoon Baek, Sehui Kim). Libya: University of Tripoli (Muhammed Elhadi), Benghazi Medical Center (Wafa Aldressi, Issa A. Abuzeid, Mohammed N. Albaraesi, Mohamed Aziz Moftah, Sarah Aldressi), Tripoli Central Hospital (Wegdan Khalel, Eman Abdulwahed, Entisar Ahmed Ali Alshareea, Akram Abdulhamid Ashur Abujrad, Reem Ghmagh, Marwa Isa Biala), National Cancer Institute Sabratha (Khawla Derwish), National Heart Center Tripoli (Rayet Al Islam Benjouira, Mohamed Aliwa), University Hospital Tripoli (Ahmed Msherghi, Ahmed Tuwaib, Tahani Mustafa, Haifa Zriba), AboSleem Hospital (Hamza Mahmoud Agilla), El-Khadra Hospital (Bahaeddin Taher Sadek Ben Hamida, Rema Hassan Mohamed Otman). Macedonia: University Hospital Skopje Mother Theresa (Maja Mojsova Mijovska). Malta: Mater Dei Hospital (Anne Marie Camilleri Podesta). Mexico: Regional Hospital of High Specialty of Ixtapaluca (Gilberto Adrián Gasca López). Palestine: Princess Alia Governmental Hospital (Sarah Amro). Portugal: Hospital São Bernardo, Central Hosptial of Setúbal (Rita de Freitas Regufe). Russia: Kemerovo Cardiology Centre (Evgeny Grigoryev, Artem Ivkin, Dmitriy Balakhnin, Dmitriy Shukevich), A.N. Bakulev National Medical Research Center of Cardiovascular Surgery (Michael Yaroustovsky). Saudi Arabia: Almana General Hospital Al-Khobar (Abdulnaser Barmou). Switzerland: University Hospital Zurich (Alexander Kaserer, Clara Castellucci, Samira Akbas). Slovenia: University Medical Centre Maribor (Andreja Möller Petrun, Irena Gregorcic, Vesna Sok). South Africa: Stellenbosch University, Faculty of Medicine and Health Sciences (Andre Links). Spain: University Hospital Virgen de las Nieves(Elizabeth Bárcena Barreto), University Hospital Infanta Leonor (Javier Ripollés Melchor), University Hospital Gran Canaria Doctor Negrín (Ángel Becerra-Bolaños, Aurelio Rodríguez-Pérez), University Hospital Son Llàtzer (Javier Mata Estévez, Juan Mulet Matas, Sara Pérez Palao), Hospital de Sant Pau (Mercedes García Álvarez, Albert Bainac Albadalejo, Astrid Batalla González, Ana María Gómez Caro, Ignacio Hinojal Blanco, Diego Toral Fernandez, Gracia Herranz Perez), Txagorritxu Hospital Álava (Margarita Logroño Ejea, Noelia de la Rosa Ruiz, María Gastaca Abasolo), San Pedro Hospital (Lourdes Ferreira, Félix Lobato, Marta Aguado Sevilla), Fundación Puigvert, (Andres Erazo), University Hospital Donostia (Berta Castellano Paulis), Hospital 12 de Octubre (Isabel de la Calle Gil), University Hospital Bellvitge (Peter Adamove, Francho Miguel Blasco Blasco), University Hospital Fundacion Alcorcón (Jose Ignacio García-Sánchez, Sara García Zamorano, Natalia Gijón Herreros), Navarra University Clinic (Raquel Callejas), University Hospital Ciudad Real (Mercedes Estaire Gómez), University Hospital Ramón y Cajal (Angel M. Candela-Toha, Elisabeth Claros-Llamas, Pilar Cobeta-Orduña, Pascual Crespo-Aliseda, Trinidad Dorado-Díaz, María Gómez-Rojo, M. Nuria Mané-Ruiz, M. Carmen Martín-González, Adolfo Martínez-Pérez, Carlos Tiscar), University Hospital Vall d’Hebron (Patricia Galán Menéndez, Verónica Estepa Calvo, Laura Llinares Espí, Yuri Santiago Loaiza Aldeán, Víctor Morales Ariza, Laura Villarino Vila), La Gerencia de Asistencia Sanitaria de Segovia (Francisco Javier García-Miguel). Sudan: Sharg Alneel Hospital and Omdurman Military Hospital (Elfayadh S. M. Suliman), Gadarif Teaching Hospital (Ahmed Mohamed Ibrahim), Ahmed Gasim Hospital (Hammad Ali Fadlalmola). Syria: Aleppo, Syria Faculty of Medicine (Sarya Swed). Taiwan: National Taiwan University Hospital and College of Medicine (Vin-Cent Wu). Turkey: Istanbul University Istanbul Faculty of Medicine (Mukadder Orhan-Sungur, Demet Altun, Nur Canbolat, Müşerref Beril Dinçer), Acibadem Mehmet Ali Aydinlar University School of Medicine (Serap Aktas Yildirim), Acibadem Mehmet Ali Aydinar University School of Medicine, Atakent Hospital (Muzeyyen Iyigun), Mersin University (Davud Yapıcı, Levent Özdemir, Aslınur Sagün), Akdeniz University (Neval Boztug, Emel Gündüz,), Cukurova University (Demet Lafli-Tunay), Ondokuz Mayis University (Deniz Karakaya, Burhan Dost, Ozgur Komurcu), Istanbul University-Cerrahpasa (Ozlem Korkmaz Dilmen, Eren Fatma Akcil, Yusuf Tunali), Manisa Celal Bayar University (Gulay Ok, Eda Tok-Alsina), Prof. Dr. Cemil Taşcıoğlu City Hospital (Cengiz Polat), Yeditepe University (Nurcan Kızılcık), University of Health Sciences Haseki Education and Training Hospital (Öznur Şen), Koç University (Kamil Darçın, Semra Uğur , Yavuz Gürkan), Health Sciences University Kartal Dr Lutfi Kirdar City Hospital (Kemal Tolga Saracoglu, Özge Yıldız-Koyuncu), Ankara Bilkent City Hospital (Z. Aslı Demir, N. Aysun Postacı, Ayşegül Özgök, Ümit Karadeniz, Hülya Yiğit Özay, Eda Balcı, Nevriye Salman, Behiç Girgin), Balikesir University (Ozlem Sagir, Hafize Fisun Demir, Fatih Ugun), Inonu University (Hüseyin İlksen Toprak), Süleyman Demirel University ( Mustafa Soner Özcan, Filiz Alkaya-Solmaz), Derince Training And Research Hospital (Mehmet Yilmaz), Bursa Yüksek Ihtisas Training and Research Hospital (Umran Karaca), Trakya University (Sevtap Hekimoglu Şahin), Ankara University İbni Sina Hospital (Süheyla Karadağ Erkoç, Neslihan Alkış), Cebeci Hospital (Volkan Baytaş), Karadeniz Technical University (Engin Erturk, Sedat Saylan, Ali Akdogan), Gazi University School of Medicine (Beyza Büyükgebiz Yeşil), Kahramanmaraş Sütçü İmam University Faculty of Medicine (Omer Faruk Boran, Yavuz Orak, Feyza Çalişir), Dokuz Eylül University (Sibel Büyükçoban, Bahar Kuvaki), Bursa Uludag University (Seda Cansabuncu, Selcan Akesen, Suna Gören), Baltalimani Metin Sabanci Bone and Joint Diseases Education and Research Hospital (Tugce Yeniocak, Osman Orman), Duzce University (Özlem Ersoy Karka), Kocaeli University (Tulay Sahin). Ukraine: Zaporizhzhia State Medical University Hospital (Natalia Momot, Anna Panchenko). USA: University of Alabama (Jean-Francois Pittet, Kristen Rutledge), Tripoli, Libya University Hospital Tripoli, Department of Anesthesiology (Ahmed Albishti, Mohamed Alsori, Salmi Hamza Abrayik).
Funding
Open Access funding enabled and organized by Projekt DEAL. The trial was supported by the German Research Foundation (KFO342-1, ZA428/18–1, and ZA428/21–1 to AZ and ME5413/1–1 to MM) and by an unrestricted research grant from Baxter (to AZ). The European Society of Anesthesiology and Intensive Care (ESAIC), among the following medical societies endorsed this study but had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript. However, we would like to thank these societies for their support on the promotion of EPIS-AKI: Colegio Mexicano de Medicina Crítica (COMMEC), Colombian Association of Surgery (CAS), Czech Society of Anesthesiology and Intensive Care (CSARIM), Deutsche Gesellschaft für Anästhesiologie und Intensivmedizin (DGAI), European Society of Anaesthesiology and Intensive Care (ESAIC), Korean Society of Anesthesiologists (KSA), Società Italiana di Anestesia Analgesia Rianimazione e Terapia Intensiva (SIAARTI), Société Française d'Anesthésie et de Réanimation (SFAR), South African Society of Anaesthesiologists (SASA), Spanish Perioperative Audit and Research Network (REDGERM), Turkish Anaesthesiology and Reanimation Society (TARD).
Author information
Authors and Affiliations
Consortia
Contributions
AZ and MM conceived and designed the study; FA and MM performed statistical analysis; RW, KR, EG, AMCT, AD, VL, PR, PGM, MGA, KP, ML, WK, MOS acquired data; AZ, RW, JAK, RB, MM drafted the manuscript; RW, KR, EG, AMCT, AD, VL, PR, PGM, MGA, KP, ML, WK, MOS made critical revision of the manuscript for key intellectual component. All authors provided final approval of the final version of the manuscript.
Corresponding author
Ethics declarations
Conflicts of interest
AZ received lecture fees from BioMerieux, Fresenius Medical Care and Baxter unrelated to current study and an unrestricted research grant from Baxter related to the current study. JAK is a paid consultant for BioMerieux, and a fulltime employee of Spectral Medical. Patricia Galán Menéndez reports fees from Baxter, Fresenius, MSD. MM received lecture fees from BioMerieux, Fresenius Medical Care and Baxter unrelated to current study. All other authors declare no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
EPIS-AKI investigators are listed in the Acknowledgements section.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Zarbock, A., Weiss, R., Albert, F. et al. Epidemiology of surgery associated acute kidney injury (EPIS-AKI): a prospective international observational multi-center clinical study. Intensive Care Med 49, 1441–1455 (2023). https://doi.org/10.1007/s00134-023-07169-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00134-023-07169-7