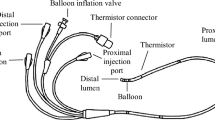
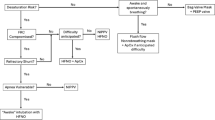
Abstract
Over the last decade, the way to monitor hemodynamics at the bedside has evolved considerably in the intensive care unit as well as in the operating room. The most important evolution has been the declining use of the pulmonary artery catheter along with the growing use of echocardiography and of continuous, real-time, minimally or totally non-invasive hemodynamic monitoring techniques. This article, which is the result of an agreement between authors belonging to the Cardiovascular Dynamics Section of the European Society of Intensive Care Medicine, discusses the advantages and limits of using such techniques with an emphasis on their respective place in the hemodynamic management of critically ill patients with hemodynamic instability.



Similar content being viewed by others
References
Connors AF Jr, McCaffree DR, Gray BA (1983) Evaluation of right-heart catheterization in the critically ill patient without acute myocardialinfarction. N Engl J Med 308:263–267
Saugel B, Ringmaier S, Holzapfel K, Schuster T, Phillip V, Schmid RM, Huber W (2011) Physical examination, central venous pressure, and chest radiography for the prediction of transpulmonary thermodilution-derived hemodynamic parameters in critically ill patients: a prospective trial. J Crit Care 26:402–410
Perel A, Saugel B, Teboul JL, Malbrain ML, Belda FJ, Fernández-Mondéjar E, Kirov M, Wendon J, Lussmann R, Maggiorini M (2015) The effects of advanced monitoring on hemodynamic management in critically ill patients: a pre and post questionnaire study. J Clin Monit Comput. doi:10.1007/s10877-015-9811-7
Gnaegi A, Feihl F, Perret C (1997) Intensive care physicians insufficient knowledge of right-heart catheterization at the bedside: time to act? Crit Care Med 25:213–220
Rajaram SS, Desai NK, Kalra A, Gajera M, Cavanaugh SK, Brampton W, Young D, Harvey S, Rowan K (2013) Pulmonary artery catheters for adult patients in intensive care. Cochrane Database Syst Rev 2:CD003408
O’Horo JC, Maki DG, Krupp AE, Safdar N (2014) Arterial catheters as a source of bloodstream infection: a systematic review and meta-analysis. Crit Care Med 42:1334–1339
Belda FJ, Aguilar G, Teboul JL, Pestaña D, Redondo FJ, Malbrain M, Luis JC, Ramasco F, Umgelter A, Wendon J, Kirov M, Fernández-Mondéjar E, PICS Investigators Group (2011) Complications related to less-invasive haemodynamic monitoring. Br J Anaesth 106:482–486
Michard F, Teboul JL (2002) Predicting fluid responsiveness in ICU patients: a critical analysis of the evidence. Chest 121:2000–2008
Vincent JL, Sakr Y, Sprung CL, Ranieri VM, Reinhart K, Gerlach H, Moreno R, Carlet J, Le Gall JR, Payen D, Sepsis Occurrence in Acutely Ill Patients (2006) Sepsis in European intensive care units: results of the SOAP study. Crit Care Med 34:344–353
Hadian M, Kim H, Severyn DA, Pinsky MR (2010) Cross-comparison of cardiac output trending accuracy of LiDCO, PiCCO FloTrac and pulmonary artery catheters. Crit Care 14:R212
Hamzaoui O, Monnet X, Richard C, Osman D, Chemla D, Teboul JL (2008) Effects of changes in vascular tone on the agreement between pulse contour and transpulmonary thermodilution cardiac output measurements within an up to 6-hour calibration-free period. Crit Care Med 36:434–440
Michard F, Boussat S, Chemla D, Anguel N, Mercat A, Lecarpentier Y, Richard C, Pinsky MR, Teboul JL (2000) Relation between respiratory changes in arterial pulse pressure and fluid responsiveness in septic patients with acute circulatory failure. Am J Respir Crit Care Med 162:134–138
Marik PE, Monnet X, Teboul JL (2011) Hemodynamic parameters to guide fluid therapy. Ann Intensive Care 1:1
Monnet X, Osman D, Ridel C, Lamia B, Richard C, Teboul JL (2009) Predicting volume responsiveness by using the end-expiratory occlusion in mechanically ventilated intensive care unit patients. Crit Care Med 37:951–956
Sakka SG, Reinhart K, Meier-Hellmann A (1999) Comparison of pulmonary artery and arterial thermodilution cardiac output in critically ill patients. Intensive Care Med 25:843–846
Monnet X, Persichini R, Ktari M, Jozwiak M, Richard C, Teboul JL (2011) Precision of the transpulmonary thermodilution measurements. Crit Care 15:R204
Gödje O, Höke K, Goetz AE, Felbinger TW, Reuter DA, Reichart B, Friedl R, Hannekum A, Pfeiffer UJ (2002) Reliability of a new algorithm for continuous cardiac output determination by pulse-contour analysis during hemodynamic instability. Crit Care Med 30:52–58
Jozwiak M, Teboul JL, Monnet X (2015) Extravascular lung water in critical care: recent advances and clinical applications. Ann Intensive Care 5:38
Cordemans C, De Laet I, Van Regenmortel N, Schoonheydt K, Dits H, Huber W, Malbrain ML (2012) Fluid management in critically ill patients: the role of extravascular lung water, abdominal hypertension, capillary leak, and fluid balance. Ann Intensive Care 5:2
Jozwiak M, Silva S, Persichini R, Anguel N, Osman D, Richard C, Teboul JL, Monnet X (2013) Extravascular lung water is an independent prognostic factor in patients with acute respiratory distress syndrome. Crit Care Med 41:472–480
Linton RA, Band DM, Haire KM (1993) A new method of measuring cardiac output in man using lithium dilution. Br J Anaesth 71:262–266
Cecconi M, Dawson D, Grounds R, Rhodes A (2009) Lithium dilution cardiac output measurement in the critically ill patient: determination of precision of the technique. Intensive Care Med 35:498–504
Slagt C, Malagon I, Groeneveld AB (2014) Systematic review of uncalibrated arterial pressure waveform analysis to determine cardiac output and stroke volume variation. Br J Anaesth 112:626–637
Critchley LA, Critchley JA (1999) A meta-analysis of studies using bias and precision statistics to compare cardiac output measurement techniques. J Clin Monit Comput 15:85–91
Hapfelmeier A, Cecconi M, Saugel B (2016) Cardiac output method comparison studies: the relation of the precision of agreement and the precision of method. J Clin Monit Comput 30:149–155
Yang X, Du B (2014) Does pulse pressure variation predict fluid responsiveness in critically ill patients? A systematic review and meta-analysis. Crit Care 18:650
Monnet X, Vaquer S, Anguel N, Jozwiak M, Cipriani F, Richard C, Teboul JL (2015) Comparison of pulse contour analysis by Pulsioflex and Vigileo to measure and track changes of cardiac output in critically ill patients. Br J Anaesth 114:235–243
Romano SM, Pistolesi M (2002) Assessment of cardiac output from systemic arterial pressure in humans. Crit Care Med 30:1834–1841
Franchi F, Silvestri R, Cubattoli L, Taccone FS, Donadello K, Romano SM, Giomarelli P, McBride WT, Scolletta S (2011) Comparison between an uncalibrated pulse contour method and thermodilution technique for cardiac output estimation in septic patients. Br J Anaesth 107:202–208
Gopal S, Do T, Pooni JS, Martinelli G (2014) Validation of cardiac output studies from the Mostcare compared to a pulmonary artery catheter in septic patients. Minerva Anestesiol 80:314–323
Dark PM, Singer M (2004) The validity of trans-esophageal Doppler ultrasonography as a measure of cardiac output in critically ill adults. Intensive Care Med 30:2060–2066
Monnet X, Chemla D, Osman D, Anguel N, Richard C, Pinsky MR, Teboul JL (2007) Measuring aortic diameter improves accuracy of esophageal Doppler in assessing fluid responsiveness. Crit Care Med 35:477–482
Hamilton MA, Cecconi M, Rhodes A (2011) A systematic review and meta-analysis on the use of preemptive hemodynamic intervention to improve postoperative outcomes in moderate and high-risk surgical patients. Anesth Analg 112:1392–1402
Marik PE (2013) Noninvasive cardiac output monitors: a state-of the-art review. J Cardiothorac Vasc Anesth 27:121–134
Saugel B, Cecconi M, Wagner JY, Reuter DA (2015) Noninvasive continuous cardiac output monitoring in perioperative and intensive care medicine. Br J Anaesth 114:562–575
Thiele RH, Bartels K, Gan TJ (2015) Cardiac output monitoring: a contemporary assessment and review. Crit Care Med 43:177–185
Saugel B, Dueck R, Wagner JY (2014) Measurement of blood pressure. Best Pract Res Clin Anaesthesiol 28:309–322
Broch O, Renner J, Gruenewald M, Meybohm P, Schottler J, Caliebe A, Steinfath M, Malbrain M, Bein B (2012) A comparison of the Nexfin(R) and transcardiopulmonary thermodilution to estimate cardiac output during coronary artery surgery. Anaesthesia 67:377–383
Chen G, Meng L, Alexander B, Tran NP, Kain ZN, Cannesson M (2012) Comparison of noninvasive cardiac output measurements using the Nexfin monitoring device and the esophageal Doppler. J Clin Anesth 24:275–283
Fischer MO, Avram R, Cârjaliu I, Massetti M, Gérard JL, Hanouz JL, Fellahi JL (2012) Non-invasive continuous arterial pressure and cardiac index monitoring with Nexfin after cardiac surgery. Br J Anaesth 109:514–521
Monnet X, Picard F, Lidzborski E, Mesnil M, Duranteau J, Richard C, Teboul JL (2012) The estimation of cardiac output by the Nexfin device is of poor reliability for tracking the effects of a fluid challenge. Crit Care 16:R212
Taton O, Fagnoul D, De Backer D, Vincent JL (2013) Evaluation of cardiac output in intensive care using a non-invasive arterial pulse contour technique (Nexfin((R))) compared with echocardiography. Anaesthesia 68:917–923
Wagner JY, Grond J, Fortin J, Negulescu I, Schofthaler M, Saugel B (2016) Continuous noninvasive cardiac output determination using the CNAP system: evaluation of a cardiac output algorithm for the analysis of volume clamp method-derived pulse contour. J Clin Monit Comput. doi:10.1007/s10877-015-9744-1
Saugel B, Meidert AS, Langwieser N, Wagner JY, Fassio F, Hapfelmeier A, Prechtl LM, Huber W, Schmid RM, Godje O (2014) An autocalibrating algorithm for non-invasive cardiac output determination based on the analysis of an arterial pressure waveform recorded with radial artery applanation tonometry: a proof of concept pilot analysis. J Clin Monit Comput 28:357–362
Wagner JY, Sarwari H, Schon G, Kubik M, Kluge S, Reichenspurner H, Reuter DA, Saugel B (2015) Radial artery applanation tonometry for continuous noninvasive cardiac output measurement: a comparison with intermittent pulmonary artery thermodilution in patients after cardiothoracic surgery. Crit Care Med 43:1423–1428
Saugel B, Reuter DA (2014) Are we ready for the age of non-invasive haemodynamic monitoring? Br J Anaesth 113:340–343
Hofer CK, Rex S, Ganter MT (2014) Update on minimally invasive hemodynamic monitoring in thoracic anesthesia. Curr Opin Anaesthesiol 27:28–35
Squara P, Denjean D, Estagnasie P, Brusset A, Dib JC, Dubois C (2007) Noninvasive cardiac output monitoring (NICOM): a clinical validation. Intensive Care Med 33:1191–1194
Kupersztych-Hagege E, Teboul JL, Artigas A, Talbot A, Sabatier C, Richard C, Monnet X (2014) Bioreactance is not reliable for estimating cardiac output and the effects of passive leg raising in critically ill patients. Br J Anaesth 111:961–966
Fagnoul D, Vincent JL, de Backer D (2012) Cardiac output measurements using the bioreactance technique in critically ill patients. Crit Care 16:460
Yamada T, Tsutsui M, Sugo Y, Sato T, Akazawa T, Sato N, Yamashita K, Ishihara H, Takeda J (2012) Multicenter study verifying a method of noninvasive continuous cardiac output measurement using pulse wave transit time: a comparison with intermittent bolus thermodilution cardiac output. Anesth Analg 115:82–87
Ball TR, Tricinella AP, Kimbrough BA, Luna S, Gloyna DF, Villamaria FJ, Culp WC Jr (2013) Accuracy of noninvasive estimated continuous cardiac output (esCCO) compared to thermodilution cardiac output: a pilot study in cardiac patients. J Cardiothorac Vasc Anesth 27:1128–1132
Biais M, Berthezene R, Petit L, Cottenceau V, Sztark F (2015) Ability of esCCO to track changes in cardiac output. Br J Anaesth 115:403–410
Thonnerieux M, Alexander B, Binet C, Obadia JF, Bastien O, Desebbe O (2015) The ability of esCCO and ECOM monitors to measure trends in cardiac output during alveolar recruitment maneuver after cardiac surgery: a comparison with the pulmonary thermodilution method. Anesth Analg 121:383–391
Biais M, Cottenceau V, Petit L, Masson F, Cochard JF, Sztark F (2011) Impact of norepinephrine on the relationship between pleth variability index and pulse pressure variations in ICU adult patients. Crit Care 15:R168
Monnet X, Guérin L, Jozwiak M, Bataille A, Julien F, Richard C, Teboul JL (2013) Pleth variability index is a weak predictor of fluid responsiveness in patients receiving norepinephrine. Br J Anaesth 110:207–213
Cannesson M, Desebbe O, Rosamel P, Delannoy B, Robin J, Bastien O, Lehot JJ (2008) Pleth variability index to monitor the respiratory variations in the pulse oximeter plethysmographic waveform amplitude and predict fluid responsiveness in the operating theatre. Br J Anaesth 101:200–206
Forget P, Lois F, de Kock M (2010) Goal-directed fluid management based on the pulse oximeter-derived pleth variability index reduces lactate levels and improves fluid management. Anesth Analg 111:910–914
Cecconi M, De Backer D, Antonelli M, Beale R, Bakker J, Hofer C, Jaeschke R, Mebazaa A, Pinsky MR, Teboul JL, Vincent JL, Rhodes A (2014) Consensus on circulatory shock and hemodynamic monitoring. Task force of the European Society of Intensive Care Medicine. Intensive Care Med 40:1785–1815
Marik PE, Cavallazzi R (2013) Does the central venous pressure predict fluid responsiveness? An updated meta-analysis and a plea for some common sense. Crit Care Med 41:1774–1781
Marik PE (2014) Iatrogenic salt water drowning and the hazards of a high central venous pressure. Ann Intensive Care 4:21
Eskesen TG, Wetterslev M, Perner A (2016) Systematic review including re-analyses of 1148 individual data sets of central venous pressure as a predictor of fluid responsiveness. Intensive Care Med 42:324–332
Pinsky MR, Kellum JA, Bellomo R (2014) Central venous pressure is a stopping rule, not a target of fluid resuscitation. Crit Care Resus 16:245–246
Wong BT, Chan MJ, Glassford NJ, Mårtensson J, Bion V, Chai SY, Oughton C, Tsuji IY, Candal CL, Bellomo R (2015) Mean arterial pressure and mean perfusion pressure deficit in septic acute kidney injury. J Crit Care 30:975–981
Squara P (2014) Central venous oxygenation: when physiology explains apparent discrepancies. Crit Care 18:579
Wetterslev M, Møller-Sørensen H, Johansen RR, Perner A (2016) Systematic review of cardiac output measurements by echocardiography vs. thermodilution: the techniques are not interchangeable. Intensive Care Med. doi:10.1007/s00134-016-4258-y
Jozwiak M, Monnet X, Teboul JL (2015) Monitoring: from cardiac output monitoring to echocardiography. Curr Opin Crit Care 21:395–401
Trof RJ, Beishuizen A, Cornet AD, de Wit RJ, Girbes AR, Groeneveld AB (2012) Volume-limited versus pressure-limited hemodynamic management in septic and nonseptic shock. Crit Care Med 40:1177–1185
Mitchell JP, Schuller D, Calandrino FS, Schuster DP (1992) Improved outcome based on fluid management in critically ill patients requiring pulmonary artery catheterization. Am Rev Respir Dis 145:990–998
Teboul JL, Monnet X, Perel A (2012) Results of questionable management protocols are inherently questionable. Crit Care Med 40:2536
Vincent JL, Pelosi P, Pearse R, Payen D, Perel A, Hoeft A, Romagnoli S, Ranieri VM, Ichai C, Forget P, Della Rocca G, Rhodes A (2015) Perioperative cardiovascular monitoring of high-risk patients: a consensus of 12. Crit Care 19:224
Scheeren TW, Wiesenack C, Gerlach H, Marx G (2013) Goal-directed intraoperative fluid therapy guided by stroke volume and its variation in high-risk surgical patients: a prospective randomized multicentre study. J Clin Monit Comput 27:225–233
Benes J, Giglio M, Brienza N, Michard F (2014) The effects of goal-directed fluid therapy based on dynamic parameters on post-surgical outcome: a meta-analysis of randomized controlled trials. Crit Care 18:584
Michard F (2016) Hemodynamic monitoring in the era of digital health. Ann Intensive Care 6:15
Maisch S, Bohm SH, Solà J, Goepfert MS, Kubitz JC, Richter HP, Ridder J, Goetz AE, Reuter DA (2011) Heart-lung interactions measured by electrical impedance tomography. Crit Care Med 39:2173–2176
Biais M, Carrié C, Delaunay F, Morel N, Revel P, Janvier G (2012) Evaluation of a new echoscopic device for focused cardiac ultrasonography in an emergency setting. Crit Care 16:R82
Drews FA, Westenskow DR (2006) The right picture is worth a thousand numbers: data displays in anesthesia. Hum Factors 48(1):59–71
Pinsky MR, Dubrawski A (2014) Gleaning knowledge from data in the ICU. Am J Respir Crit Care Med 190:606–610
De Backer D, Donadello K, Sakr Y, Ospina-Tascon G, Salgado D, Scolletta S, Vincent JL (2013) Microcirculatory alterations in patients with severe sepsis: impact of time of assessment and relationship with outcome. Crit Care Med 41:791–799
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
JLT is a member of the medical advisory board of Pulsion Medical Systems and received honoraria from Edwards Lifesciences and Masimo Inc. for consulting. BS is a member of the medical advisory board of Pulsion Medical Systems and a received institutional research grants, unrestricted research grants, and refunds of travel expenses from Tensys Medical Inc. BS received honoraria for giving lectures for CNSystems Medizintechnik AG. MC consulted and lectured for Edwards Lifesciences and LiDCO. He received support from Edwards Lifesciences, LiDCO, Deltex Medical, Applied Physiology, Masimo, Bmeye, Cheetah Medical, Imacor (travel expenses, honoraria, advisory board, unrestricted educational grant, and research material). DDB received honoraria for lectures for Edwards Lifesciences and Nihon Kohden. DDB received grant/material for studies for Edwards Lifesciences, Maquet, Vytech, Cheetah, Imacor, and Nihon Kohden. XM is a member of the medical advisory board of Pulsion Medical systems and received honoraria from Cheetah Medical for consulting. AP is a member of the medical advisory board of Pulsion Medical Systems and is a consultant for Masimo Inc. MRP is a consultant for Edwards Lifesciences, Masimo Inc., and LiDCO and has stock options in LiDCO and Cheetah Medical companies. DAR is a member of the medical advisory board of Pulsion Medical Systems and gave lectures for Edwards Lifesciences. AR has no conflict of interest to declare. PS was a consultant for Cheetah Medical and for Edwards Lifesciences. TS received honoraria from Edwards Lifesciences and Masimo Inc. for consulting. TS received honoraria from Pulsion Medical Systems for lecturing. JLV has no conflict of interest to declare.
Additional information
On behalf of the Cardiovascular Dynamics Section of the European Society of Intensive Care Medicine (ESICM).
Rights and permissions
About this article
Cite this article
Teboul, JL., Saugel, B., Cecconi, M. et al. Less invasive hemodynamic monitoring in critically ill patients. Intensive Care Med 42, 1350–1359 (2016). https://doi.org/10.1007/s00134-016-4375-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00134-016-4375-7