Abstract
Purpose
Diaphragm function should be monitored in critically ill patients, as full ventilatory support rapidly induces diaphragm atrophy. Monitoring the electrical activity of the diaphragm (EAdi) may help assess the level of diaphragm activity, but such monitoring results are difficult to interpret because reference values are lacking. The aim of this study was to describe EAdi values in critically ill children during a stay in the pediatric intensive care unit (PICU), from the acute to recovery phases, and to assess the impact of ventilatory support on EAdi.
Methods
This was a prospective longitudinal observational study of children requiring mechanical ventilation for ≥24 h. EAdi was recorded using a validated method in the acute phase, before extubation, after extubation, and before PICU discharge.
Results
Fifty-five critically ill children were enrolled in the study. Median maximum inspiratory EAdi (EAdimax) during mechanical ventilation was 3.6 [interquartile range (IQR) 1.2–7.6] μV in the acute phase and 4.8 (IQR 2.0–10.7) μV in the pre-extubation phase. Periods of diaphragm inactivity (with no detectable inspiratory EAdi) were frequent during conventional ventilation, even with a low level of support. EAdimax in spontaneous ventilation was 15.4 (IQR 7.4–20.7) μV shortly after extubation and 12.6 (IQR 8.1–21.3) μV before PICU discharge. The difference in EAdimax between mechanical ventilation and post-extubation periods was significant (p < 0.001). Patients intubated mainly because of a lung pathology exhibited higher EAdi (p < 0.01), with a similar temporal increase.
Conclusions
This is the first systematic description of EAdi evolution in children during their stay in the PICU. In our patient cohort, diaphragm activity was frequently low in conventional ventilation, suggesting that overassistance or oversedation is common in clinical practice. EAdi monitoring appears to be a helpful tool to detect such situations.
Similar content being viewed by others
Introduction
Ventilatory support is one of the main reasons for admission into the pediatric intensive care unit (PICU). One-third of these patients need ventilation for >4 days, leading to a prolonged stay in the PICU and high healthcare costs [1]. Mechanical ventilation and critical illness can rapidly induce severe diaphragmatic dysfunction and atrophy [2, 3]. This secondary respiratory muscle dysfunction contributes to weaning prolongation and should be monitored and prevented [4, 5]. Overassistance should therefore be avoided—not only to limit ventilator induced lung injury, but also to keep the diaphragm active [6]. Insufficient support may also be detrimental as it may lead to respiratory muscle fatigue [7] that can delay recovery. Optimal ventilatory assistance should therefore be reached, but little data are available in clinical practice to guide the adjustment of the support. In addition, the sedation can also influence the respiratory drive and diaphragm activity [8].
The electrical activity of the diaphragm (EAdi) can now be continuously monitored in critically ill adults [9–11] and children [12–14]. The EAdi signal is used not only to control the degree of assistance provided by the neurally adjusted ventilatory assist (NAVA), a mode of mechanical ventilation [15], but EAdi signal also provides the clinician with a bedside evaluation of respiratory drive and diaphragm activity [4, 8, 10, 16].
We hypothesized that episodes of insufficient or excessive ventilatory assist could be detected by following the evolution of diaphragm activity during a patient’s stay in the PICU. As this technology has only recently become available, reference EAdi levels in critically ill children admitted to the PICU have not yet been determined. The objectives of this study were (1) to describe the inspiratory EAdi levels in critically ill children during a PICU stay, from the acute phase to the PICU discharge, and (2) to assess the impact of ventilation on inspiratory EAdi. Parts of the results were presented at the ATS conference in 2012 [17].
Patients and methods
This prospective observational study was conducted in the PICU of Sainte-Justine Hospital from August 2010 to October 2012. The study was approved by the local Ethics committee and registered on controlled-trials.com. Written informed consent was obtained from the patients’ parents.
Consecutive children aged <18 years admitted to the PICU and requiring invasive mechanical ventilation for >24 h were eligible for entry into the study. The minimal age required for inclusion was initially >30 days, and subsequently amended to >7 days in December 2011 to increase recruitment. Patients were included when evidence of spontaneous breathing was present. Exclusion criteria are detailed in the Electronic Supplementary Material (ESM).
Study protocol
The EAdi signal was recorded at four time-points during two phases of invasive ventilation and two phases of ventilation without the endotracheal tube.
-
Acute phase A 30-min recording was obtained as soon as the patient’s respiratory frequency was consistently above the ventilator minimal rate set in absence of autotriggering.
-
Pre-extubation EAdi was recorded for 15 min in the 4 h preceding extubation.
-
Post-extubation EAdi was recorded for 15 min in the 2 h following extubation.
-
PICU discharge a 15-min recording of EAdi was conducted in the 2 h prior PICU discharge, or when the removal of the EAdi catheter was planned because respiratory status was normal.
The last three recordings were made only if the EAdi catheter was still in place at these time-points.
EAdi signal acquisition and analysis
Details on EAdi recordings and analysis are provided in the ESM. Briefly, EAdi was recorded using a specific nasogastric tube (NAVA catheter; Maquet, Solna, Sweden) and a dedicated Servo I ventilator (v6.0; Maquet) [16, 18], while the ventilation was continued without any modification. For each recording, EAdi was analyzed by two investigators to identify neural inspiratory and expiratory times during a 5-min period.
Clinical data recorded
Clinical and laboratory data were prospectively recorded at baseline and prior to each recording, as detailed in the ESM.
Statistical methods
Inter-observer agreement for the EAdi analysis between two investigators was assessed with intraclass correlation coefficients (ICCs). Provided there was a good inter-observer reproducibility (i.e., ICC > 0.75 [19]), each investigator’s data were averaged for subsequent data analysis. Repeated-measures analysis of variance (ANOVA) was used to compare variables in the four phases with Fisher’s least significant difference (LSD) or Games-Howell post hoc tests for group differences, when appropriate. Linear regression analyses were conducted to evaluate the association between clinical data and peak EAdi in the acute and the final phase, as detailed in ESM. A two-tailed p value of <0.05 was considered to be significant. Data are presented as the median with the interquartile range (IQR).
Results
Characteristics of the population
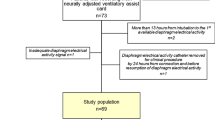
During the study period, 406 patients admitted to the PICU met eligibility criteria. As illustrated in ESM Fig. 1, 32 patients never reached inclusion criteria, and 314 patients had at least one exclusion criteria. Sixty patients were included in the study, but no recordings could be made before extubation in five of them for technical reasons. The 55 remaining patients were included in the analysis. The median age of the eligible patients who were not included was 8 (IQR 1–48) months, similar to that of the analyzed patients (p = 0.96).
Among the 55 analyzed patients, recordings were available during the acute phase in 52 patients, 23 in the pre-extubation period, 26 in the post-extubation period, and 23 at PICU discharge. Patient characteristics are presented in Table 1. No significant difference was observed between the group characteristics.
Description of diaphragm electrical activity
A total of 25,912 breaths were analyzed, with a median of 192 (IQR 136–279) breaths per recordings. Inter-observer agreement of EAdi data was excellent, with ICCs of 0.84, 0.92, 0.85, and 0.87 for neural inspiratory time, expiratory time, inspiratory peak EAdi, and mean inspiratory EAdi, respectively.
The EAdi characteristics for the four periods are reported in Table 2 and Fig. 1. Compared to children without a tracheal tube, in those with a tracheal tube the inspiratory EAdi during the acute phase was significantly lower (p < 0.01, repeated-measures ANOVA), with significant differences between the acute phase and the two post-extubation periods, and between the pre- and post-extubation periods. Low levels of EAdi (peak EAdi < 2 μV) were frequent in the mechanical ventilation phase [33 % of patients during the acute phase, 25 % of patients in pre-extubation period vs. 1 patient (4 %) after extubation]. We also observed frequent “silences” (no detectable inspiratory effort based on EAdi signal) during mechanical ventilation: for 15 (29 %) patients there was no inspiratory EAdi signal on more than one-third of the acute phase recordings (Table 2). The evolution of EAdi in a representative patient is shown in Fig. 2.
Evolution of peak inspiratory electrical activity of the diaphragm (EAdi) during the stay in the pediatric intensive care unit (PICU). Diaphragm activity was significantly lower during the acute phase than in the post-extubation and final recordings, and lower during the pre-extubation phase than during the post-extubation period (p < 0.01, repeated-measures ANOVA; p < 0.05, post hoc comparisons). Shaded box Interquartile range (IQR; 25th–75th percentile), thick horizontal bar median, vertical interval (whiskers) interval with 95 % of data, circles data outside the limits of the vertical bars
Evolution of EAdi (blue lines) and airway pressure waveforms (red lines) in a representative critically ill child in the acute phase [a, with pressure-support ventilation (PSV) of 7 cmH2O/PEEP (positive end expiratory pressure) of 5 cmH2O), in the pre-extubation period (b, with PSV of 7 cmH2O/PEEP of 5 cmH2O), in the post-extubation period (c, with spontaneous breathing), and before discharge from the PICU (d, with spontaneous breathing)
Inspiratory EAdi was higher in children intubated for a respiratory disease when compared to other reasons (Fig. 3; p < 0.01). In the subgroup of patients for whom four recordings were available, the evolution of inspiratory EAdi followed a similar pattern (ESM Fig. 4).
Relationship between clinical condition and EAdi
The magnitude of ventilatory support (as reflected by driving pressure, i.e., the difference between end-inspiratory and end-expiratory pressures) has no observable influence on acute phase inspiratory EAdi (p = 0.6; ESM Fig. 2), with some patients having low EAdi even with a driving pressure of <10 cmH2O. There was also no observable impact of ventilatory mode on EAdi (p = 0.6; ESM Fig. 3). A non-significant trend for a lower EAdi was observed in patients with lower COMFORT scores (p = 0.09; ESM Fig. 2). No association was observed between EAdi and the time since the last bolus, or between EAdi and the dose of benzodiazepine or opioids received in the 4 h (all R 2 < 0.01; ESM Fig. 5). In the univariate analysis as in the multivariate analysis, the respiratory distress as a cause for intubation was the only factor associated with EAdi in the acute phase (p < 0.05). Age, gender, and COMFORT scale were not independently associated with EAdi (p = 0.13, 0.6, and 0.21, respectively).
At PICU discharge, peak EAdi was higher in patients with bronchiolitis (p < 0.01) and seemed to be negatively correlated with age (p < 0.05). In the multivariate analysis, bronchiolitis diagnosis was the only variable associated with EAdi (p < 0.01), while age, PELOD (Pediatric Logistic Organ Dysfunction) score, and respiratory distress as a reason for intubation were not independent factors of EAdi (p = 0.21, 0.34, and 0.47, respectively).
Extubation event
Inspiratory EAdi increased significantly from the pre- to post-extubation period (Table 2). In the 18 patients for whom both recordings were available, inspiratory EAdi increased by 116 % (IQR 25–218 %) from the pre-extubation level to the post-extubation period. A single patient exhibited a decrease in EAdi (by 11 %). Six patients (12 %) failed the first extubation attempt and had to be re-intubated. Pre-extubation peak EAdi was available in only three of these patients and was 1, 12, and 43 μV, respectively.
Discussion
We describe the evolution of EAdi in critically ill children from the acute phase to PICU discharge. EAdi was frequently blunted during the mechanical ventilation phase when compared to the spontaneous breathing phase. Extubation was followed by an EAdi increase, suggesting a work of breathing augmentation.
Diaphragm electrical activity in spontaneously breathing children
Tools for EAdi monitoring are now available in clinical practice and may benefit the patient by facilitating the diagnosis of patient–ventilator asynchrony [9, 13, 18, 20, 21], central hypoventilation and apneas [22, 23], the titration of ventilatory support [16, 24, 25], evolution of diaphragm injury [16, 26, 27], and assessment of neuro-ventilatory efficiency during weaning [28–30]. However, EAdi reference values are lacking, making it difficult to interpret a recorded EAdi value. EAdi values can theoretically be interpreted in relation to the maximal EAdi obtained during a voluntary maximal effort [10] or during a standardized trial with minimal support [25]. However, voluntary maximal inspiration can not reliably be obtained in critical conditions, and the definition of minimal support is not clear in the PICU.
The EAdi values we recorded in our pediatric patients prior to PICU discharge provide a landmark by which to interpret EAdi values, even if an interpretation of the data in relation to the patient’s own EAdi evolution makes more sense than a comparison to a different group. These patients were recovering from a critical illness and although their EAdi may differ from that of healthy children, their condition was stable and they were ready to be transferred to the ward. Therefore, the EAdi observed at this stage is likely representative of children in whom the effort of breathing is not strikingly elevated. Similar reference data have not been reported in adult patients. In 17 non-intubated preterm infants, Stein et al. [31] reported a peak EAdi of 11 ± 4 mcV, with no significant relation to gestational age. Those EAdi values are relatively close to our observed values. We also did not observe a relationship between age and EAdi, but the majority of patients were infants.
Those patients who had been intubated mainly due to a respiratory disease had higher EAdi, but this difference tended to be smaller prior PICU discharge, reflecting the improvement in ventilation status. Importantly, patients with neuromuscular disorder were not included, and the results should not be extrapolated to these conditions. Myopathy-affected patients likely have a different pattern, usually with a high EAdi to compensate for the low muscle efficiency during the earlier stage of the disease, and later with a low EAdi when muscular degeneration is advanced [27, 32]. Patients with central hypoventilation have a low EAdi [27].
Diaphragm electrical activity in mechanically ventilated children
We observed that the EAdi was markedly lower during ventilation than in extubated children. This is not a surprising result as the aim of mechanical ventilation is to decrease the effort involved in the work of breathing. However, an unexpected finding was the high prevalence of severely blunted diaphragm activity. Such events have been reported by Alander et al. [33] in 15 children. In this study, EAdi was absent in 8 ± 10 % and 12 ± 18 % of the time during pressure-triggered and flow-triggered ventilation, respectively, as compared to 1 ± 3 % during NAVA ventilation. Our results confirm that periods of low EAdi are frequent and prolonged during conventional ventilation. Several reasons can explain this inactivity of the diaphragm.
First, the timing of the recordings may be important. Periods during which neuromuscular blockade or deep sedation was used were not eligible for assessment in our study, and evident spontaneous breaths were needed as inclusion criteria. The first recording was obtained a median of 3 (IQR 1–7) days after intubation. EAdi in the pre-extubation period was only slightly higher than in the acute phase, and periods without activity were not shorter in the latter, suggesting that low diaphragm activity is not a temporary event, but is frequent during conventional ventilation.
Second, the sedative treatment can certainly impact diaphragm activity. Deeply sedated patients were excluded from the assessment, but most patients received some kind of sedation, with a rather low comfort scale. However, this level of sedation is usually observed in our PICU. No major improvement of EAdi was observed between the acute phase and pre-extubation period when sedation was decreased, and low levels of diaphragm activity were also observed in patients with a higher COMFORT-B score. Vaschetto et al. [8] showed that in mechanically ventilated adults sedation can reduce the ventilatory drive. These authors found that the reduction was more important during ventilation with fixed pressure support, while during neurally adjusted ventilation the ventilatory drive was less affected, suggesting a synergistic impact of sedation when associated with overassistance.
Third, respiratory activity is influenced by the magnitude of assistance [24, 34]. This is well illustrated by the striking increase of EAdi between pre- and post- extubation. Excessive ventilatory support likely explains part of our results. Based on clinical observation, blood gases, and ventilator settings, excessive assistance was not suspected prior to the study, but only evidenced by the observation of low EAdi. The absence of a clear relationship between the level of support and EAdi observed between subjects can be explained by differences in patient conditions. A given level of assistance has a different impact on ventilatory drive depending on the respiratory compliance and CO2 drive. As illustrated in Fig. 2, EAdi can be markedly decreased with little support providing the lungs are healthy. The potential of overassistance to decrease ventilatory drive has also been described in adults [8, 24]. In addition, the level of support varies with patient effort and is dependent on the ventilatory mode [35]. In the absence of esophageal pressure monitoring, the support quantification is limited. Our results should therefore be seen as an average result of common ventilation practices, but the assessment of the precise association between EAdi and ventilatory support deserves further studies in experimental and controlled design. The ventilatory mode and the synchrony could also influence EAdi. Indeed, the reduction in trigger delay with NAVA has been shown to decrease the effort associated with the work of breathing [35, 36]. No patient in this study was recorded in NAVA ventilation, and no difference of EAdi pattern was observed depending on the ventilatory mode.
Fourthly, endotracheal tube is sometimes perceived as an important additional resistance, which would require an increased work of breathing (or assist) to overcome. However, several studies have shown that the work of breathing decreases with intubation [37, 38] and that the need of support to compensate for a tracheal tube is likely to be minimal [39]. It seems unlikely however that tracheal intubation alone can explain the low ventilatory drive.
Lastly, patient disease could also result in low EAdi. Perturbations in the neuromuscular function or the ventilatory respiratory center can impact EAdi [22, 23, 26, 40, 41], but no such patients were included in our study. Respiratory depression following viral infection is classical, and we observed some apneas in that context, but most patients with viral pneumonia or bronchiolitis showed a rather high level of EAdi.
All of the factors mentioned above (timing, disease, level and type of support and sedation, intubation) may have contributed to our results, but it is important to note that in this representative cohort of PICU patients, periods with low EAdi were frequent and not recognized. Full mechanical support is associated with diaphragm atrophy in adults [2, 3]. This may be similar in neonates [42], but pediatric data are lacking. The high prevalence of diaphragm inactivity could generate a similar disorder in children. The use of EAdi targets to titrate the respiratory support has been proposed in adults [25] and children [43]. While our results support the pertinence of implementing such a strategy and also provide additional information for the choice of EAdi target, the benefit of the titration method needs further validation.
Change in diaphragmatic activity after extubation
The EAdi markedly increased after extubation. The pre-extubation ventilatory settings were not standardized but can be considered to be relatively minimal (median driving pressure of 8 cmH2O). This suggests that such support is sufficient to blunt the ventilatory drive. In contrasting to common belief, such settings in pre-extubation do not accurately reflect the post-extubation efforts, supporting the expert-based recommendation that extubation readiness should be tested without inspiratory support [39].
As diaphragm function is crucial for successful weaning, diaphragm neuro-ventilatory efficiency, as reflected by the ratio of tidal volume to EAdi, may be a marker of interest for the evaluation of extubation readiness. The authors of a pediatric study reported contradictory results [44], but the results were difficult to interpret: the patients were assisted with various levels of support levels, the ventilator contribution being difficult to predict. In adults with no support, NVE seems to be a good predictor of extubation success [28, 29].
Study limitations
There are a number of limitations to our study. The recruitment process was complex, and many patients could not be included. However, the patient characteristics accurately reflect our usual PICU population. The small sample size did not enable our results to be stratified according to patient age. The medical conditions of the patients varied considerably; however, our results should not be generalized to conditions not studied, especially neuromuscular diseases. The study was conducted in a single center. We did not measure the work of breathing and transpulmonary pressure, which limited our ability to assess the ventilatory assist. Plateau pressures were measured without an inspiratory pause, which could have resulted in an overestimation. Future research on the correlation between the work of breathing and EAdi needs to be conducted.
Conclusions
We describe EAdi levels in a cohort of critically ill children admitted to the PICU, with frequent periods of blunted diaphragm activity. We recommend that greater attention be paid to excessive assistance during conventional ventilation. EAdi monitoring facilitates the detection of these events and could help in adjusting the support given to such patients and minimizing the risk of diaphragm atrophy.
References
Payen V, Jouvet P, Lacroix J, Ducruet T, Gauvin F (2012) Risk factors associated with increased length of mechanical ventilation in children. Pediatr Crit Care Med 13:152–157
Jaber S, Petrof BJ, Jung B, Chanques G, Berthet JP, Rabuel C, Bouyabrine H, Courouble P, Koechlin-Ramonatxo C, Sebbane M, Similowski T, Scheuermann V, Mebazaa A, Capdevila X, Mornet D, Mercier J, Lacampagne A, Philips A, Matecki S (2011) Rapidly progressive diaphragmatic weakness and injury during mechanical ventilation in humans. Am J Respir Crit Care Med 183:364–371
Levine S, Nguyen T, Taylor N, Friscia ME, Budak MT, Rothenberg P, Zhu J, Sachdeva R, Sonnad S, Kaiser LR, Rubinstein NA, Powers SK, Shrager JB (2008) Rapid disuse atrophy of diaphragm fibers in mechanically ventilated humans. N Engl J Med 358:1327–1335
Doorduin J, van Hees HW, van der Hoeven JG, Heunks LM (2013) Monitoring of the respiratory muscles in the critically ill. Am J Respir Crit Care Med 187:20–27
Matamis D, Soilemezi E, Tsagourias M, Akoumianaki E, Dimassi S, Boroli F, Richard JC, Brochard L (2013) Sonographic evaluation of the diaphragm in critically ill patients. Technique and clinical applications. Intensive Care Med 39:801–810
Brochard L, Harf A, Lorino H, Lemaire F (1989) Inspiratory pressure support prevents diaphragmatic fatigue during weaning from mechanical ventilation. Am Rev Respir Dis 139:513–521
Roussos C, Macklem PT (1982) The respiratory muscles. N Engl J Med 307:786–797
Vaschetto R, Cammarota G, Colombo D, Longhini F, Grossi F, Giovanniello A, Della Corte F, Navalesi P (2014) Effects of propofol on patient—ventilator synchrony and interaction during pressure support ventilation and neurally adjusted ventilatory assist. Crit Care Med 42:74–82
Colombo D, Cammarota G, Alemani M, Carenzo L, Barra FL, Vaschetto R, Slutsky AS, Della Corte F, Navalesi P (2011) Efficacy of ventilator waveforms observation in detecting patient-ventilator asynchrony. Crit Care Med 39:2452–2457
Sinderby C, Beck J, Spahija J, Weinberg J, Grassino A (1998) Voluntary activation of the human diaphragm in health and disease. J Appl Physiol 85:2146–2158
Sinderby CA, Beck JC, Lindstrom LH, Grassino AE (1997) Enhancement of signal quality in esophageal recordings of diaphragm EMG. J Appl Physiol 82:1370–1377
Beck J, Reilly M, Grasselli G, Mirabella L, Slutsky AS, Dunn MS, Sinderby C (2009) Patient-ventilator interaction during neurally adjusted ventilatory assist in low birth weight infants. Pediatr Res 65:663–668
Beck J, Tucci M, Emeriaud G, Lacroix J, Sinderby C (2004) Prolonged neural expiratory time induced by mechanical ventilation in infants. Pediatr Res 55:747–754
Emeriaud G, Beck J, Tucci M, Lacroix J, Sinderby C (2006) Diaphragm electrical activity during expiration in mechanically ventilated infants. Pediatr Res 59:705–710
Sinderby C, Navalesi P, Beck J, Skrobik Y, Comtois N, Friberg S, Gottfried SB, Lindstrom L (1999) Neural control of mechanical ventilation in respiratory failure. Nat Med 5:1433–1436
Ducharme-Crevier L, Du Pont-Thibodeau G, Emeriaud G (2013) Interest of monitoring diaphragmatic electrical activity in the pediatric intensive care unit. Crit Care Res Pract 2013:384210
Emeriaud G, Massicotte E, Ducharme-Crevier L, Beck J, Jouvet P (2012) Diaphragm activity monitoring : a new tool to assess the impact of ventilatory support. Am J Respir Crit Care Med 185:A2343
Bordessoule A, Emeriaud G, Morneau S, Jouvet P, Beck J (2012) Neurally adjusted ventilatory assist improves patient-ventilator interaction in infants as compared with conventional ventilation. Pediatr Res 72:194–202
Szklo M, Nieto F (1999) Epidemiology: beyond the basics. Aspen Publishers Inc., Gaithersburg, pp 479–482
Vignaux L, Grazioli S, Piquilloud L, Bochaton N, Karam O, Jaecklin T, Levy-Jamet Y, Tourneux P, Jolliet P, Rimensberger PC (2013) Optimizing patient-ventilator synchrony during invasive ventilator assist in children and infants remains a difficult task. Pediatr Crit Care Med 14:e316–e325
Piquilloud L, Vignaux L, Bialais E, Roeseler J, Sottiaux T, Laterre PF, Jolliet P, Tassaux D (2011) Neurally adjusted ventilatory assist improves patient-ventilator interaction. Intensive Care Med 37:263–271
Rahmani A, Ur Rehman N, Chedid F (2013) Neurally adjusted ventilatory assist (NAVA) mode as an adjunct diagnostic tool in congenital central hypoventilation syndrome. J Coll Physicians Surg Pak 23:154–156
Szczapa T, Beck J, Migdal M, Gadzinowski J (2013) Monitoring diaphragm electrical activity and the detection of congenital central hypoventilation syndrome in a newborn. J Perinatol 33:905–907
Colombo D, Cammarota G, Bergamaschi V, De Lucia M, Corte FD, Navalesi P (2008) Physiologic response to varying levels of pressure support and neurally adjusted ventilatory assist in patients with acute respiratory failure. Intensive Care Med 34:2010–2018
Roze H, Lafrikh A, Perrier V, Germain A, Dewitte A, Gomez F, Janvier G, Ouattara A (2011) Daily titration of neurally adjusted ventilatory assist using the diaphragm electrical activity. Intensive Care Med 37:1087–1094
Bordessoule A, Emeriaud G, Delnard N, Beck J, Jouvet P (2010) Recording diaphragm activity by an oesophageal probe: a new tool to evaluate the recovery of diaphragmatic paralysis. Intensive Care Med 36:1978–1979
Fine-Goulden MR, Puppala NK, Durward A (2012) Mechanisms of ventilator dependence in children with neuromuscular and respiratory control disorders identified by monitoring diaphragm electrical activity. Intensive Care Med 38:2072–2079
Liu L, Liu H, Yang Y, Huang Y, Liu S, Beck J, Slutsky AS, Sinderby C, Qiu H (2012) Neuroventilatory efficiency and extubation readiness in critically ill patients. Crit Care 16:R143
Roze H, Repusseau B, Perrier V, Germain A, Seramondi R, Dewitte A, Fleureau C, Ouattara A (2013) Neuro-ventilatory efficiency during weaning from mechanical ventilation using neurally adjusted ventilatory assist. Br J Anaesth 111:955–960
Dres M, Schmidt M, Ferre A, Mayaux J, Similowski T, Demoule A (2012) Diaphragm electromyographic activity as a predictor of weaning failure. Intensive Care Med 38:2017–2025
Stein H, Hall R, Davis K, White DB (2013) Electrical activity of the diaphragm (Edi) values and Edi catheter placement in non-ventilated preterm neonates. J Perinatol 33:707–711
Beck J, Weinberg J, Hamnegard CH, Spahija J, Olofson J, Grimby G, Sinderby C (2006) Diaphragmatic function in advanced Duchenne muscular dystrophy. Neuromuscul Disord 16:161–167
Alander M, Peltoniemi O, Pokka T, Kontiokari T (2012) Comparison of pressure-, flow-, and NAVA-triggering in pediatric and neonatal ventilatory care. Pediatr Pulmonol 47:76–83
Beck J, Gottfried SB, Navalesi P, Skrobik Y, Comtois N, Rossini M, Sinderby C (2001) Electrical activity of the diaphragm during pressure support ventilation in acute respiratory failure. Am J Respir Crit Care Med 164:419–424
Heulitt MJ, Clement KC, Holt SJ, Thurman TL, Jo CH (2012) Neurally triggered breaths have reduced response time, work of breathing, and asynchrony compared with pneumatically triggered breaths in a recovering animal model of lung injury. Pediatr Crit Care Med 13:e195–e203
Clement KC, Thurman TL, Holt SJ, Heulitt MJ (2011) Neurally triggered breaths reduce trigger delay and improve ventilator response times in ventilated infants with bronchiolitis. Intensive Care Med 37:1826–1832
Keidan I, Fine GF, Kagawa T, Schneck FX, Motoyama EK (2000) Work of breathing during spontaneous ventilation in anesthetized children: a comparative study among the face mask, laryngeal mask airway and endotracheal tube. Anesth Analg 91:1381–1388
Willis BC, Graham AS, Yoon E, Wetzel RC, Newth CJ (2005) Pressure-rate products and phase angles in children on minimal support ventilation and after extubation. Intensive Care Med 31:1700–1705
Newth CJ, Venkataraman S, Willson DF, Meert KL, Harrison R, Dean JM, Pollack M, Zimmerman J, Anand KJ, Carcillo JA, Nicholson CE (2009) Weaning and extubation readiness in pediatric patients. Pediatr Crit Care Med 10:1–11
Liet JM, Dejode JM, Joram N, Gaillard Le Roux B, Pereon Y (2013) Bedside diagnosis of bilateral diaphragmatic paralysis. Intensive Care Med 39:335
Roze H, Richard JC, Mercat A, Brochard L (2011) Recording of possible diaphragm fatigue under neurally adjusted ventilatory assist. Am J Respir Crit Care Med 184:1213–1214
Knisely AS, Leal SM, Singer DB (1988) Abnormalities of diaphragmatic muscle in neonates with ventilated lungs. J Pediatr 113:1074–1077
Kallio M, Peltoniemi O, Anttila E, Pokka T, Kontiokari T (2014) Neurally adjusted ventilatory assist (NAVA) in pediatric intensive care—a randomized controlled trial. Pediatr Pulmonol. doi: 10.1002/ppul.22995
Wolf GK, Walsh BK, Green ML, Arnold JH (2011) Electrical activity of the diaphragm during extubation readiness testing in critically ill children. Pediatr Crit Care Med 12:e220–e224
Acknowledgments
The authors are indebted to the patients and their families for their willingness to participate in our study. We thank Mariana Dumitrascu, Laurence Bertout, and Noémie Loron for their help in the screening and enrolment process, Nicole Poitras for the study management support, the respiratory therapists for their logistic help, the PICU fellows and attending healthcare providers for their collaboration, and Norman Comtois for his support regarding signal recording and analysis. The study was supported by a Young Investigator Award of the Respiratory Health Network of the Fonds de la Recherche du Québec–Santé and by an operating grant for applied clinical research of CHU Sainte-Justine and Sainte-Justine Research Center. Neurovent research Inc. provided a recording device. Maquet Critical Care provided the ventilator and catheters for the study.
Conflicts of interest
GE, AL, LDC, EM, OF, AAPL, SM, and PJ have no conflict of interest. JB has been reimbursed by Maquet Critical Care (Solna, Sweden) for attending several conferences; JB has participated as a speaker in scientific meetings or courses organized and financed by Maquet Critical Care; JB, through Neurovent Research, serves as a consultant to Maquet Critical Care. The following disclosure was agreed upon by University of Toronto, Sunnybrook Health Sciences Centre, St-Michael’s Hospital and the REBs of Sunnybrook and St-Michael’s to resolve conflicts of interest: “Dr. Beck has made inventions related to neural control of mechanical ventilation that are patented. The patents are assigned to the academic institution(s) where inventions were made. The license for these patents belongs to Maquet Critical Care. Future commercial uses of this technology may provide financial benefit to Dr. Beck through royalties. Dr. Beck owns 50 % of Neurovent Research Inc. (NVR). NVR is a research and development company that builds the equipment and catheters for research studies. NVR has a consulting agreement with Maquet Critical Care”. St-Michael’s Hospital has a research agreement with Maquet Critical Care AB (Solna, Sweden) and receives royalty and overhead from this agreement.
Author information
Authors and Affiliations
Corresponding author
Additional information
Take-home message: As compared to the diaphragm electrical activity (EAdi) observed in non-intubated conditions, EAdi is lower during pediatric conventional mechanical ventilation, with frequent periods of blunted diaphragm activity. EAdi monitoring facilitates the detection of these events and could guide the adjustment of the ventilatory support in order to minimize the risk of diaphragm atrophy.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Emeriaud, G., Larouche, A., Ducharme-Crevier, L. et al. Evolution of inspiratory diaphragm activity in children over the course of the PICU stay. Intensive Care Med 40, 1718–1726 (2014). https://doi.org/10.1007/s00134-014-3431-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00134-014-3431-4