Abstract
Introduction
Critically ill patients often develop acute lung injury (ALI) in the context of different clinical conditions. We aimed to explore differences in early local and systemic features in three experimental animal models of ALI.
Methods
Mechanically ventilated male Sprague–Dawley rats were randomized to high tidal volume (VT) ventilation (HVT) (n = 8, VT 24 ml/kg), massive brain injury (MBI) (n = 8, VT 8 ml/kg) or endotoxemia (LPS) (n = 8, VT 8 ml/kg). Each experimental group had its own control group of eight rats (VT 8 ml/kg). We measured arterial blood gases, mean arterial pressure, lung compliance, inflammatory mediators in plasma and their expression and gelatinase activity in the lungs after 3 h of injury.
Results
Despite maintaining relatively normal lung function without evidence of important structural changes, we observed altered lung and systemic inflammatory responses in all three experimental models. LPS triggered the most robust inflammatory response and HVT the lowest systemic proinflammatory response. The HVT group had higher Il6, Tnf and Cxcl2 mRNA in lungs than MBI animals. Metalloproteinase activity/expression and neutrophilic recruitment in the lungs were higher in HVT than in LPS or MBI.
Conclusions
The early responses to direct or remote lung insult in our three models of ALI captured different physiological and biological features that could lead to respiratory and/or multiorgan failure.
Similar content being viewed by others
Background
Several pulmonary and non-pulmonary clinical conditions could predispose critically ill patients with healthy lungs to develop acute lung injury (ALI) and the acute respiratory distress syndrome (ARDS) [1]. The clinical presentation of both syndromes includes severe hypoxemia and alterations in lung function that result from increased lung permeability because of compromised alveolo-capillary integrity; ALI/ARDS is also accompanied by local or distant inflammation [1]. The degree of lung injury can vary depending upon whether it is caused by a direct insult to the lung or by an external agent, such as lipopolysaccharide (LPS) or inflammatory mediators from other organs that reach the lung through the systemic circulation. However, the mechanisms of lung injury, time courses, inflammatory pathways and cell repair processes are not well understood.
Recently, Matute-Bello et al. [2] described the strengths and weaknesses of different pulmonary- and extrapulmonary-induced ALI models commonly used to reproduce the physiologic and cellular effects of human ALI in animals. There is no consensus as to what exactly constitutes ALI in an animal, in part because there is no single marker or parameter that has both sufficient sensitivity and specificity to identify the occurrence of all forms and severities of ALI [2–4]. Since the mechanisms through which the inflammatory response to different stimuli takes place remain unclear, we hypothesized that those inflammatory mediators would predispose lungs to ALI, even prior to worsening of lung function. We aimed to characterize early features in three experimental conditions known to cause ALI in previously healthy lungs. The first model consisted of applying high tidal volume (VT) ventilation (HVT) to promote the translation of cyclic mechanical stretch into biological responses at the epithelial-cellular level (i.e., ventilator-induced lung injury; VILI) [5–7]. The second model consisted of inducing massive brain injury (MBI), which combines an ischemic insult with the effect of the catecholamine release in the context of mechanical ventilation (MV), concurrently affecting lung endothelium and epithelium [8]. The third model consisted of administering intravenous LPS, which represents a direct endothelial insult similar to that observed in patients with sepsis [9]. Our findings suggest that even in the absence of florid lung dysfunction immediately after the 3-h period after injury, all these hits will contribute to biotrauma and cause ALI.
Materials and methods
Animal preparation
All procedures and techniques were approved by the Animal Ethics Committee of the Hospital de Sabadell (Barcelona, Spain). Forty-eight adult male Sprague–Dawley rats weighing 350–370 g housed in standard conditions with food and water ad libitum were anesthetized with intramuscular ketamine (75 mg/kg, Parke-Davis, El Prat de Llobregat, Spain) and xylazine (10 ml/kg, Rompun® Bayer, Barcelona, Spain), tracheotomized and paralyzed with intravenous pancuronium bromide (2 ml/kg, Organon, Cornellá de Llobregat, Spain). An endotracheal tube (2-mm inner diameter) was inserted and tightly tied to avoid air leaks, and the rats were ventilated using a Servo 300 ventilator (Siemens, Solna, Sweden). VT was set and measured using the ventilator’s pneumotachograph. Airway pressure was monitored via a side port in the tracheal tube using a pressure transducer (Valydine MP45, Valydine Engineering, Northridge, CA). The left carotid artery was cannulated and connected to a pressure transducer (Transpac Monitoring Kit; Abbot, Sligo, Ireland). The right jugular vein was cannulated for fluid infusion. Blood and airway pressures were routed to an amplifier (Presograph, Gould Godart, Netherlands), converted to digital (Urelab, Barcelona, Spain) and recorded in a personal computer (Anadat-Labdat Software, RTH InfoDat, Montreal, Canada.
Experimental protocol
At baseline, all animals were ventilated in volume-controlled ventilation mode with 8 ml/kg VT and 2 cmH2O positive end-expiratory pressure (PEEP). Inspired oxygen fraction (FiO2) was kept at 0.4 throughout the experiment, and respiratory rate was fixed for normocapnia. Thirty minutes after surgical preparation, baseline values of arterial blood gases and respiratory system parameters were measured. Then, rats were randomly assigned to one of the six experimental groups: three models of ALI and three respective control groups (8 rats per group): (1) HVT group, which received 24 ml/kg VT and 0 cmH2O PEEP (instrumental dead space was increased to maintain normocapnia without decreasing respiratory rate); (2) MBI group; (3) intravenous LPS group. The baseline ventilatory pattern was maintained in MBI and LPS animals and in all controls. Lung mechanics variables were measured hourly. Arterial blood gases were measured at the end of the experimental period. Fluid management was identical in all groups (Ringer-lactate, 10 ml/kg/h) to prevent differences that might favor edema formation with the exception of LPS and its control, which received a bolus (0.5 ml) of fluid as the LPS vehicle. Vasoactive drugs were not used in any group. At the end of the 3-h period, rats were euthanatized by exsanguination. Seven milliliters of blood withdrawn from each animal was centrifuged and the plasma was stored at −80°C for protein determinations. The rats’ hearts and lungs were removed en bloc, and the right lung was frozen for additional tissue analyses.
Induction of massive brain injury
MBI was induced by inflating an intracranial balloon-tipped catheter with saline solution to raise intracranial pressure above 150 mmHg [8]. This maneuver causes herniation of the brain stem and electrocerebral silence in 20 min [10]. We verified MBI by confirming intracranial pressure continuously higher than mean arterial pressure and dilated, fixed pupils without photomotor reflex. We did not perform electroencephalography. No craniotomies were performed in controls.
LPS administration
LPS from E. coli 055:B5 serotype (Sigma–Aldrich, Spain) was dissolved in sterile saline and administered intravenously in a single bolus at a dose of 4 mg/kg.
Hemodynamic and lung function measurements
Arterial blood gases, systemic arterial pressures (MAP) and respiratory system mechanics (inspiratory and expiratory pause) were recorded at baseline and hourly to calculate static lung compliance (Crs).
Histological analysis
Left lungs were fixed by instillation of 4% buffered formaldehyde into the airway at a pressure of 5 cmH2O and immersed in the same fixative. Histological scores after hematoxylin–eosin (HE) staining were calculated as previously described by two investigators who were blinded to experimental groups [11]. Neutrophil intraalveolar infiltration was blindly assessed by counting the number of neutrophils in 50 fields per animal at a magnification of 400× using ImageJ v1.36 (Wayne Rasband, NIH, USA).
Plasma protein immunoassays
To elucidate the changes in biomarker levels and patterns associated with each experimental model, we used a semiquantitative protein array system for rat cytokines (RayBio Rat Cytokine Antibody Array, RayBiotech, Inc., Norcross, GA). After this approach, commercially available enzyme-linked immunosorbent assay (ELISA) kits (Biosource, Camarillo, CA) were used to determine the following plasma protein levels: tumor necrosis factor-alpha (TNF)-α, chemokine (C–X–C motif) ligand 2 (a.k.a. macrophage inflammatory protein or MIP-2), interleukin (IL)-6, chemokine (C–C motif) ligand 2 (a.k.a. monocyte chemoattractant protein or MCP-1) and IL-10. Analyses of all samples, standards and controls were run in duplicate.
Tissue mRNA analysis
We studied mRNA expression of matrix metalloproteinase (Mmp)2, Mmp9, Timp1 (a.k.a. Mmp inhibitor 1), Il6, Tnf, chemokine C–X–C motif ligand 2 (Cxcl2) and chemokine (C–C motif) ligand 2 (Ccl2) genes by the real-time PCR method detailed in ESM.
Lung tissue gelatinase activity: gelatin zymography
MMP-2 and MMP-9 activities in lung tissue were determined by densitometric analysis of gelatin zymograms with TL100 software (nonlinear dynamics) (method detailed in ESM).
Statistical analysis
All values are expressed as mean ± SEM. All data were tested for homogeneity of variance (Levene’s test), and, when required, variables were logarithmically transformed to achieve homogeneity of variances. Ratios (fold changes) indicating magnitude of response with respect to controls or between groups were compared using analysis of variance (ANOVA). Groups were compared using one-way ANOVA with the Bonferroni adjustment for multiple comparisons or using a nonparametric test (Kruskal–Wallis) for variables without homogeneity of variances. Comparisons of gas exchange, mechanics and hemodynamics data were performed using a two-way ANOVA with the main factors time (repeated measures) and group. Level of significance was set at p < 0.05.
Results
All the raw data are provided in the electronic supplementary material (ESM) (Tables 1S, 2S, 3S; Fig. 1S). Animal body weights were similar in all groups. At baseline, no differences in hemodynamics or gas exchange were observed between groups.
Physiological variables
MAP remained stable and in the normal range throughout the 3 h in all groups (Fig. 1). Only a significant trend toward decreased MAP was found in MBI with respect to its controls. Respiratory system compliance (Crs) remained unchanged throughout the experimental period in controls and LPS animals. HVT increased airway pressure (ESM) and Crs significantly (Fig. 1).
Fold changes in hemodynamics, lung mechanics and gas exchange with respect to controls. MAP remained stable in all groups, with a minimal trend toward a decrease in the MBI group (*p < 0.05 vs. control). Crs increased significantly in the HVT group (†p < 0.05 vs. MBI and HVT). Data are presented as mean ± SE. MAP mean arterial pressure, Crs respiratory system compliance, CRL control, HVT high tidal volume, MBI massive brain injury, LPS lipopolysaccharide
Significant decreases in PaO2 and concurrent increases in PaCO2 compared with baseline values were found in all animals after 3 h (Fig. 1).
Histology
Figure 2 shows representative images of lungs in each experimental group. HVT led to a significant increase in neutrophil trapping in the airspace, alveolar hemorrhage and edema. In contrast, LPS administration and MBI promoted neutrophil entrapping in the pulmonary capillaries, but only slightly in the alveolar space (p = 0.09 and p = 0.3, respectively, vs. controls) (Fig. 2).
Microscopic images of lungs of representative animals in each model (a) and fold changes in alveolar neutrophil counts versus controls (b). Histological analysis evaluating lung parenchyma with hematoxylin–eosin staining. HVT increased alveolar hemorrhage and neutrophil infiltration. Original magnification ×400. CRL control group, HVT high tidal volume ventilation, MBI massive brain injury, LPS lipopolysaccharide
Inflammatory mediators
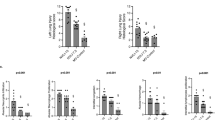
Plasma levels of IL-6, TNFα, IL-10, MCP-1 and MIP-2 are shown in Fig. 3. Plasma levels of IL-6 and IL-10 were higher in HVT, MBI and LPS than in their respective controls (ESM). Plasma TNFα increased in LPS (p = 0.04), but remained unaltered in HVT and MBI. Plasma MCP-1 and MIP-2 were unaltered by HVT. Only plasma MIP-2 increased in the MBI group (p = 0.04), while plasma MCP-1 and MIP-2 increased in LPS (p = 0.006, p < 0.0001, respectively) (Fig. 3, ESM).
Fold changes in proinflammatory proteins in each model with respect to its controls. The proinflammatory response observed in LPS is much more robust than in the other two models. Data are presented as mean ± SE. *p < 0.05 versus the experimental group’s own control group. CRL control, HVT high tidal volume, MBI massive brain injury, LPS lipopolysaccharide, IL interleukin, TNF tumor necrosis factor, MCP monocyte chemotactic protein, MIP macrophage-inflammatory protein
Measurements of cytokine expression in the lungs are shown in Fig. 4 and Table 2S ESM. Lung Il6 mRNA increased significantly versus controls in HVT (p = 0.002) and LPS (p = 0.04), but in MBI the increase was not significant. Tnf mRNA remained unaltered in HVT and MBI, but increased in LPS (p = 0.006) (Fig. 4, ESM). Compared to their respective controls, HVT and LPS had significantly higher Cxcl2 lung chemokine gene expression, whereas MIP-2 lung chemokine expression tended to decrease in MBI. Moreover, Ccl2 increased significantly compared with controls only in LPS (Fig. 4, ESM). Comparing groups on the fold changes with respect to their controls, HVT had a higher response than MBI for Il6 mRNA (p = 0.024). On the other hand, LPS had the greatest increase in Tnf and Cxcl2 lung expression, followed by HVT, while MBI values appear unaltered. Moreover, Ccl2 increased more in LPS than in HVT and MBI (Fig. 4). HVT did not alter Mmp2, Mmp9 or Timp1 lung gene expression (ESM). In contrast, LPS significantly increased Timp1, but decreased Mmp2, whereas MBI decreased Mmp9 lung gene expression (ESM).
Fold changes in lung local expression of proinflammatory mediators in each model with respect to its controls. The expression of proinflammatory mediators was lowest in MBI. Data are presented as mean ± SE. *p < 0.05 versus the experimental group’s own control group. CRL control, HVT high tidal volume, MBI massive brain injury, LPS lipopolysaccharide, Il interleukin, TNF tumor necrosis factor, CCl2 chemokine (C–C motif) ligand 2, Cxcl2 chemokine (C–X–C motif) ligand 2 macrophage-inflammatory protein
The Mmp2/Timp1 mRNA ratio was maintained in HVT, but significantly decreased in MBI and LPS (Fig. 5). However, MMP-9 gelatinase activity significantly decreased in MBI compared to its control (Fig. 5, ESM), and the Mmp9/Timp1 ratio was significantly lower in LPS and MBI than in HVT.
Fold changes in lung local gelatinase activity and expression ratios for remodeling mediators in each model with respect to its controls. Top: MMP-2 gelatinase activity is upregulated in HVT, and Mmp/Timp-1 ratios are downregulated in MBI and LPS with respect to their controls. Bottom: Gelatin zymogram of a representative animal from each model. Data are presented as mean ± SE. *p < 0.05 versus the experimental group’s own control group. CRL control, HVT high tidal volume, MBI massive brain injury, LPS lipopolysaccharide, MMP matrix metalloproteinases, TIMP matrix metalloproteinases inhibitor
Discussion
We used three well-known animal models of ALI to examine which early acting local or systemic inflammatory mediators could be involved in predisposing critically ill patients to ALI. We found an early robust systemic and lung inflammatory response to LPS, in good agreement with a generalized response to endotoxin. By contrast, the inflammatory response to MBI and HVT was moderate to weak. The inflammatory response triggered in our three models was not paralleled by a concurrent lung dysfunction since gas-exchange and lung mechanics did not deteriorate significantly within the 3-h frame. The lack of functional lung alterations in the three models could also be attributed to the use of only one hit in each, since we applied HVT in healthy lungs but used protective MV in MBI or LPS animals. Alternatively, it is plausible that our 3-h period was too short for lung dysfunction to develop. In animal systems that seek to model ALI, maximal injury is usually evident within 4–24 h of exposure to the inciting stimulus [2]. Whether lung condition would worsen with time as a consequence of the injurious stimuli alone or acting synergistically with MV remains to be determined.
HVT ventilation involves direct tissue damage due to overstretching of alveolar walls resulting in endothelial/epithelial breaks and interstitial edema and hemorrhage [12]. HVT caused an altered inflammatory response mediated by Il6 and Cxcl2 expression in the lung and was accompanied by an increase in circulating IL-6 and IL-10 proteins. TNF-α, IL-6 and IL-8, or their equivalent (MIP-2/KC in rodents) are among the mediators triggering the inflammatory response to heightened strain most consistently associated with VILI in healthy lungs [12–18]. In our study, we did not detect significant changes in Tnf levels after HVT, probably because its expression is transient, peaking early and disappearing thereafter, while Cxcl2 expression is more sustained, as is corroborated in our study. Chemokines promote lung leukocyte recruitment, infiltration and activation, all key steps in the self-amplifying inflammatory cascade [19].
Our findings may partially explain why different authors have presented discrepant results on VILI models, depending on whether they used healthy or pre-injured animals: an injurious ventilatory pattern could affect healthy or pre-inflamed lungs differently, and the inflammatory stimulus is necessary to induce further injury during MV [4, 20]. Since lungs in critically ill patients are often affected by inflammatory stimuli from remote, non-pulmonary disease processes, we used the MBI and LPS models to investigate the effects of these stimuli on the lung. We used the MBI model because recent reports link brain with lung injuries [21–23], and several authors have described respiratory failure in patients with severe head injury. In our study, MBI increased both lung and systemic inflammation in rats with intact lung function. We also found in a rabbit model that MBI primed the lungs for subsequent injury by decreasing pulmonary tolerance to stresses like MV and ischemia reperfusion [8]. The mechanisms involved in the brain-lung interaction that trigger lung injury remain unclear, but the inflammatory response seems to have a central role. The massive sympathetic discharge after MBI promotes endothelial damage and increases lung permeability [24, 25]. In our MBI model, only subtle changes in inflammatory cytokines without affecting lung structure or function were observed. After brain death, MCP-1 and IL-6 increase in the heart, lung and kidney, while Tnf is upregulated in the lung [26]. Fisher et al. [27] reported that high IL-8 levels in donor lungs were associated to early graft failure. In MBI, we found high levels of Cxcl2, the rodent equivalent of IL-8. This non-uniform pattern of cytokine expression in MBI may cause variable priming that would condition the development of ALI in different degrees [26].
We observed a clear inflammatory response in the endotoxemia model, including up-regulation of genes involved in inflammation and a systemic release of inflammatory proteins. Mediators in response to different stimuli found in the bloodstream probably arise from multiple sources, including the lung. Our findings in the LPS model, especially those regarding MIP-2, showed interesting similarities with those of other studies, suggesting that MIP-2 up-regulation might play a role in the pathogenesis of ALI. LPS enhances production of MIP-2 in rat lung explants [28]. Herrera et al. [29] and Altemeier et al. [30, 31] described the putatively deleterious synergy between MV and endotoxemia in an animal model. Despite the differences in the experimental setup, these studies identify MIP-2, or TNF-α and IL-6, as possible key mediators of the synergistic action between MV and endotoxemia.
LPS administration is followed by neutrophil entrapment in pulmonary capillaries, but only small numbers of neutrophils migrate to the air spaces, preceding subsequent alterations in the permeability of the alveolar/epithelial barrier [2]. LPS and MBI promote systemic inflammation that could prime alveolar macrophages a posteriori, but initially, they do not cause severe endothelial and epithelial damage. On the other hand, overstretching of alveolar walls produces endothelial and epithelial breaks, favoring neutrophil migration to the air spaces [2]. Despite the apparent contrast between the increase in inflammatory mediators such as MIP-2 and pulmonary neutrophil counts in the LPS and HVT models, LPS should be considered a direct stimulus for neutrophils and other immune and non-immune cells, whereas the inflammatory response triggered by HVT is not as direct on these cells, so that fewer but highly activated cells might produce larger amounts of inflammatory mediators.
In our study, ventilatory function was not severely compromised in any group. Recent studies have emphasized the role of the integrity of the extracellular matrix in the development of ALI [32]. We also assessed well-known biomarkers that contribute to maintaining or remodeling lung structure. Lung connective tissue displays a dynamic balance between ongoing breakdown/remodeling, and MMP’s activity determines the rate of repair of the alveolar epithelium. Different authors conclude that HVT drives the system to dynamic remodeling [32, 33]. The ratios between lung mRNA encoding for gelatinases and their naturally occurring main tissue inhibitor, Timp1, were down-regulated in both LPS and MBI, but unaltered in HVT. These findings suggest that the physiologically beneficial remodeling of the lung parenchyma in healthy conditions is disturbed in LPS and MBI [33, 34].
The current study has limitations that prevent extrapolation to the clinical setting. Differences in animal instrumentation, variability among the control groups, the dosage and route of LPS administration, as well as the timing of tissue sampling and exposure to each insult, might be crucial for interpreting our data and for comparing our results with those from other studies. However, animal models can help defining the mechanisms involved, since animals and humans share many physiological processes. Although critically ill patients are highly monitored, the early cellular and molecular alterations may well appear before any meticulous clinical monitoring system could detect changes in lung function. Knowledge about these molecular patterns could elucidate the mechanisms by which responses to subsequent stimuli are (inappropriately) amplified, exacerbating pulmonary injury and compromise of remote organ function. The early identification of markers of this silent inflammatory response would allow a finer and earlier intervention in the critically ill before poor lung mechanics, gas exchange or hemodynamics is evident at the bedside.
Conclusions
Even when clinically available variables, including MAP, gas exchange and lung mechanics, are within the normal ranges, some subclinical signs of an altered lung and/systemic inflammatory response or organ remodeling might be present. The inflammatory response from different pulmonary and extrapulmonary conditions that predispose to ALI compromises many different components. Crosstalk between biomarkers involved in the inflammatory and remodeling responses could be responsible for maintaining tissue integrity. Further studies are warranted to elucidate the role of these molecules during the early stages of critical illness that predispose to ALI.
References
Ware LB, Matthay MA (2000) The acute respiratory distress syndrome. N Engl J Med 342:1334–1349
Matute-Bello G, Frevert CW, Martin T (2008) Animal models of acute lung injury. Am J Physiol Lung Cell Mol Physiol 295:379–399
Menezes SL, Bozza PT, Neto HC, Laranjeira AP, Negri EM, Capelozzi VL, Zin WA, Rocco PR (2005) Pulmonary and extrapulmonary acute lung injury: inflammatory and ultrastructural analyses. J Appl Physiol 98:1777–1783
Lewis CC, Yang JY, Huang X, Banerjee SK, Blackburn MR, Baluk P, McDonald DM, Blackwell TS, Nagabhushanam V, Peters W, Voehringer D, Erle DJ (2008) Disease-specific gene expression profiling in multiple models of lung disease. Am J Respir Crit Care Med 177:376–387
Dos Santos CC, Slutsky AS (2006) The contribution of biophysical lung injury to the development of biotrauma. Annu Rev Physiol 68:585–618
Slutsky AS (2005) Ventilator-induced lung injury: from barotrauma to biotrauma. Respir Care 50:646–659
Imai Y, Parodo J, Kajikawa O, de Perrot M, Fischer S, Edwards V, Cutz E, Liu M, Keshavjee S, Martin TR, Marshall JC, Ranieri VM, Slutsky AS (2003) Injurious mechanical ventilation and end-organ epithelial cell apoptosis and organ dysfunction in an experimental model of acute respiratory distress syndrome. JAMA 289:2104–2112
López-Aguilar J, Villagrá A, Bernabé F, Murias G, Piacentini E, Real J, Fernández-Segoviano P, Romero PV, Hotchkiss JR, Blanch L (2005) Massive brain injury enhances lung damage in an isolated lung model of ventilator-induced lung injury. Crit Care Med 33:1077–1083
Bregeon F, Delpierre S, Chetaille B, Kajikawa O, Martin TR, Autillo-Touati A, Jammes Y, Pugin J (2005) Mechanical ventilation affects lung function and cytokine production in an experimental model of endotoxemia. Anesthesiology 102:331–339
Takada M, Nadeau KC, Hancock WW, Mackenzie HS, Shaw GD, Waaga AM, Chandraker A, Sayegh MH, Tilney NL (1998) Effects of explosive brain death on cytokine activation of peripheral organs in the rat. Transplantation 65:1533–1542
López-Aguilar J, Piacentini E, Villagra A, Murias G, Pascotto S, Saenz-Valiente A, Fernandez-Segoviano P, Hotchkiss JR, Blanch L (2006) Contributions of vascular flow and pulmonary capillary pressure to ventilator-induced lung injury. Crit Care Med 34:1106–1112
Villar J, Herrera-Abreu MT, Valladares F, Muros M, Pérez-Méndez L, Flores C, Kacmarek RM (2009) Experimental ventilator-induced lung injury: exacerbation by positive end-expiratory pressure. Anesthesiology 110:1341–1347
Pugin J (2003) Molecular mechanisms of lung cell activation induced by cyclic stretch. Crit Care Med 31:200–206
Wilson MR, Choudhury S, Goddard ME, O’Dea KP, Nicholson AG, Takata M (2003) High tidal volume upregulates intrapulmonary cytokines in an in vivo mouse model of ventilator-induced lung injury. J Appl Physiol 95:1385–1393
Haitsma JJ, Ulig S, Göggel R, Verbrugge SJ, Lachmann U, Lachmann B (2000) Ventilator-induced lung injury leads to loss of alveolar and systemic compartmentalization of tumor necrosis factor-alpha. Intensive Care Med 26:1515–1522
Tremblay LN, Miatto D, Hamid Q, Govindarajan A, Slutsky AS (2002) Injurious ventilation induces widespread pulmonary epithelial expression of tumor necrosis factor-alpha and interleukin-6 messenger RNA. Crit Care Med 30:1693–1700
Dreyfuss D, Ricard JD, Saumon G (2003) On the physiologic and clinical relevance of lung-borne cytokines during ventilator-induced lung injury. Am J Respir Crit Care Med; 167:1467–1471
Nonas SA, Moreno-Vinasco L, Ma SF, Jacobson JR, Desai AA, Dudek SM, Flores C, Hassoun PM, Sam L, Ye SQ, Moitra J, Barnard J, Grigoryev DN, Lussier YA, Garcia JG (2007) Use of consomic rats for genomic insights into ventilator-associated lung injury. Am J Physiol Lung Cell Mol Physiol 293:L292–L302
Belperio JA, Keane MP, Burdick MD, Londhe V, Xue YY, Li K, Phillips RJ, Strieter RM (2002) Critical role for CXCR2 and CXCR2 ligands during the pathogenesis of ventilator-induced lung injury J. Clin Invest 110:1703–1716
Tremblay LN, Slutsky AS (2005) Pathogenesis of ventilator-induced lung injury: trials and tribulations. Am J Physiol Lung Cell Mol Physiol 288:596–598
Gonzalvo R, Martí-Sistac O, Blanch L, López-Aguilar J (2007) Bench-to-bedside review: brain-lung interaction in the critically ill—a pending issue revisited. Crit Care 11:216
Yildirim E, Kaptanoglu E, Ozisik K, Beskonakli E, Okutan O, Sargon MF, Kilinc K, Sakinci U (2004) Ultrastructural changes in pneumocyte type II cells following traumatic brain injury in rats. Eur J Cardiothorac Surg 25:523–529
Yildirim E, Solaroglu I, Okutan O, Ozisik K, Kaptanoglu E, Sargon MF, Sakinci U (2004) Ultrastructural changes in tracheobronchial epithelia following experimental traumatic brain injury in rats: protective effect of erythropoietin. J Heart Lung Transplant 23:1423–1429
Chiari P (2000) Biphasic response after brain death induction: prominent part of catecholamines release in this phenomenon. J Hearth Lung Transplant 19:675–682
Avlonitis VS, Wigfield CH, Golledge HD, Kirby JA, Dark JH (2007) Early hemodynamic injury during donor brain death determines the severity of primary graft dysfunction after lung transplantation. Am J Transplant 7:83–90
Skrabal CA, Thompson LO, Potapov EV, Southard RE, Joyce DL, Youker KA, Noon GP, Loebe M (2005) Organ-specific regulation of pro-inflammatory molecules in heart, lung, and kidney following brain death. J Surg Res 123:118–125
Fisher AJ, Donnelly SC, Hirani N, Haslett C, Strieter RM, Dark JH, Corris PA (2001) Elevated levels of interleukin-8 in donor lungs is associated with early graft failure after lung transplantation. Am J Respir Crit Care Med 163:259–265
Isowa N, Liu M (2001) Role of LPS-induced microfilament depolymerization in MIP-2 production from rat pneumocytes. Am J Physiol Lung Cell Mol Physiol 280:762–770
Herrera MT, Toledo C, Valladares F, Muros M, Díaz-Flores L, Flores C, Villar J (2003) Positive end-expiratory pressure modulates local and systemic inflammatory responses in a sepsis-induced lung injury model. Intensive Care Med 29:1345–1353
Altemeier WA, Matute-Bello G, Gharib SA, Glenny RW, Martin TR, Liles WC (2005) Modulation of lipopolysaccharide-induced gene transcription and promotion of lung injury by mechanical ventilation. J Immunol 175:3369–3376
Altemeier WA, Matute-Bello G, Frevert CW, Kawata Y, Kajikawa O, Martin TR, Glenny RW (2004) Mechanical ventilation with moderate tidal volumes synergistically increases lung cytokine response to systemic endotoxin. Am J Physiol Lung Cell Mol Physiol 287:533–542
Lanchou J, Corbel M, Tanguy M, Germain N, Boichot E, Theret N, Clement B, Lagente V, Malledant Y (2003) Imbalance between matrix metalloproteinases (MMP-9 and MMP-2) and tissue inhibitors of metalloproteinases (TIMP-1 and TIMP-2) in acute respiratory distress syndrome patients. Crit Care Med 31:536–542
Foda HD, Rollo EE, Drews M, Conner C, Appelt K, Shalinsky DR, Zucker S (2001) Ventilator-induced lung injury upregulates and activates gelatinases and EMMPRIN: attenuation by the synthetic matrix metalloproteinase inhibitor, Prinomastat (AG3340). Am J Respir Cell Mol Biol 25:717–724
Kim JH, Suk MH, Yoon DW, Lee SH, Hur GY, Jung KH, Jeong HC, Lee SY, Lee SY, Suh IB, Shin C, Shim JJ, In KH, Yoo SH, Kang KH (2006) Inhibition of matrix metalloproteinase-9 prevents neutrophilic inflammation in ventilator-induced lung injury. Am J Physiol Lung Cell Mol Physiol 291:580–587
Acknowledgments
The authors would like to thank Neus Gómez (supported by FIS CA 05/0138) and Milagros Martínez for their technical assistance. The study was supported by Plan Nacional de Investigación Científica, Desarrollo e Innovación Tecnológica and Instituto de Salud Carlos III—FIS PI-04/2365, Ministerio de Educación y Ciencia BFU2006-07124/BFI, Fundació Parc Taulí CIR. JLA received support from the Programa de Estabilización FIS and Health Department of the Generalitat de Catalunya. CF was supported by a specific agreement between the Instituto de Salud Carlos III and FUNCIS (EMER07/001) under the ENCYT 2015 framework.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
López-Aguilar, J., Quilez, M.E., Martí-Sistac, O. et al. Early physiological and biological features in three animal models of induced acute lung injury. Intensive Care Med 36, 347–355 (2010). https://doi.org/10.1007/s00134-009-1695-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00134-009-1695-x