Abstract
Objective
Controversy surrounds the use of parenteral nutrition in critical illness. Previous overviews used composite scales to identify high-quality trials, which may mask important differences in true methodological quality. Using a component-based approach this meta-analysis investigated the effect of trial quality on overall conclusions reached when standard enteral nutrition is compared to standard parenteral nutrition in critically ill patients.
Methods
An extensive literature search was undertaken to identify all eligible trials. We retrieved 465 publications, and 11 qualified for inclusion. Nine trials presented complete follow-up, allowing the conduct of an intention to treat analysis.
Results
Aggregation revealed a mortality benefit in favour of parenteral nutrition, with no heterogeneity. A priori specified subgroup analysis demonstrated the presence of a potentially important treatment-subgroup interaction between studies of parenteral vs. early enteral nutrition compared to parenteral vs. late enteral. Six trials with complete follow-up reported infectious complications. Infectious complications were increased with parenteral use. The I2 measure of heterogeneity was 37.7%.
Conclusions
Intention to treat trials demonstrated reduced mortality associated with parenteral nutrition use. A priori subgroup analysis attributed this reduction to trials comparing parenteral to delayed enteral nutrition. Despite an association with increased infectious complications, a grade B+ evidence-based recommendation (level II trials, no heterogeneity) can be generated for parenteral nutrition use in patients in whom enteral nutrition cannot be initiated within 24 h of ICU admission or injury.
Similar content being viewed by others
Introduction
Parenteral nutrition (PN) has been in common use since the 1960s [1] and is accepted as the standard of care for patients with chronic non-functioning gastrointestinal tracts [2, 3]. The appropriate use of PN in the intensive care unit (ICU), however, is somewhat more controversial [4, 5].
A recent survey revealed that, depending on country of admission, 19–71% of patients received PN as their sole source of nutritional support at some time during their ICU stay [6]. The promotion of high-quality evidence has been shown to result in a more consistent approach to the provision of nutritional support, which led to improved patient outcomes [7].
Although the results obtained from well conducted level I studies often disagree with the findings of meta-analyses based on level II trials [8], rigorously conducted systematic reviews can be used to support clinical decision making [9]. The strength of the conclusions reached by a systematic review, however, are intimately related to the quality of the individual trials included in it [10]. The importance of considering individual trial quality can be illustrated by contrasting the results of two recent meta-analyses.
The Cochrane Injuries Group Albumin Reviewers’ [11] meta-analysis reported treatment with human serum albumin increased the risk of death compared with either crystalloids or no treatment. This meta-analysis included numerous pseudo-randomised trials, which are known to be subject to allocation bias [12]. A subsequent meta-analysis which specifically addressed individual components of trial methodological quality found that when only high-quality trials were considered, there was no significant effect of albumin use on overall mortality [13]. The inclusion of trials of low methodological quality is known to have a substantial impact on the conclusions reached by meta-analyses [14].
Previous systematic reviews of nutritional support interventions [15, 16, 17, 18], have identified high-quality studies using composite methodological quality scales [19] which combine different dimensions of trial quality into an overall summary score. Most composite scales are known to have used weak methodology when selecting items for inclusion and ranking them for relative importance [20].
The composite scale selected to reflect overall trial quality can dramatically influence the conclusions of a meta-analysis [21]. Because composite scales may mask important differences in true methodological quality, the use of a ‘component approach’ has been recommended [10, 22]. A component approach assesses key methodological dimensions individually, without the calculation of a summary score [14].
The purpose of this meta-analysis was to use a component approach to investigate the effect of methodological quality on the overall conclusions reached when trials comparing the use of standard PN to standard enteral nutrition (EN) in critically ill patients were aggregated.
Materials and Methods
Literature search
Medline (http://www.PubMed.org) and EMBASE (http://www.Ovid.com) were cross-searched using sensitive (broad) search statements [23] customised to each engine to detect all controlled trials, overviews and evidence-based guidelines of primary feeding interventions. Reference lists of overviews and evidence-based guidelines were hand searched. Experts and Industry representatives were contacted in order to ensure trials were not missed. The final closeout date for the search process was 30 April 2003.
Study selection
All controlled trials comparing primary feeding interventions and published in the English language [14, 24] were reviewed. Only truly randomised trials comparing standard EN to standard PN and reporting clinically meaningful outcomes were eligible. Standard EN and PN solutions were defined as any solution not supplemented with additional glutamine, arginine or other immune enhancing ingredients. PN was defined as an intravenous solution containing protein and a source of non-protein energy with or without lipids [18].
Because previous overviews detected heterogeneity between critically ill and non-critically ill patient populations [18], an explicit, objective definition of a critically ill patient population was applied [25]. Trials conducted in non-critically ill patient populations were not considered for inclusion. A study was determined to have been conducted in a critically ill patient population if the manuscript reported one of the following:
-
The patients were recruited in an ICU.
-
The inclusion criteria described were such that the patients would normally be cared for in an ICU (e.g. all patients were receiving invasive mechanical ventilatory support).
-
The patients were suffering from a condition that usually requires care in an ICU (e.g. severe thermal burns of >40–50% total body surface area, multi-trauma that required urgent laparotomy).
-
The patients had an average ICU length of stay longer than 2 days.
-
A majority of the patients received a therapy that is delivered in the ICU (e.g. invasive mechanical ventilation).
-
A severity of illness score was reported that was commensurate with the patients being critically ill.
True methodological quality
Methodological quality was based on the reporting of three key methodological components in the published manuscript: (a) presentation of an intention to treat (ITT) analysis, (b) maintenance of allocation concealment during randomisation and (c) appropriate use of blinding (e.g. participants, investigators, outcome adjudicators) [14, 22].
To investigate the effect of trial quality on overall conclusions, a series of meta-analyses were planned. The initial meta-analysis considered trials that addressed all three dimensions of quality, followed by meta-analyses based on each component individually.
To address the impact of incomplete follow-up a sensitivity analysis was undertaken. The primary meta-analysis was conducted on all trials presenting complete follow-up. Since some degree of random loss to follow-up may occur, complete follow-up was defined as full reporting on at least 95% of all patients. The sensitivity analysis was conducted including trials reporting loss to follow-up by study arm, where the total loss did not exceed 10% of all patients. The conservative assumption that the lost patient encountered an undesirable outcome was made.
The presence of excessive loss to follow-up, defined as loss of more than 10% of patients, was considered a major methodological flaw [26, 27]. Trials with excessive loss to follow-up were not eligible for consideration [28].
Any differences in interpretation were resolved by discussion.
A priori defined subgroup analysis
A treatment-subgroup interaction was investigated for trials comparing early EN initiation to PN and trials comparing late EN initiation to PN. Early EN was defined as feeding within 24 h of ICU admission or initial injury [7].
Outcomes assessed
A clinically meaningful outcome was defined as a direct measure of how a patient feels, functions or survives [29]. Any trial explicitly reporting clinically meaningful outcomes (e.g. a validated quality of life instrument, duration of survival, quality-adjusted survival or landmark mortality) was considered for inclusion. Trials reporting only unvalidated surrogate outcomes were not eligible for inclusion [30].
Previous reviewers have suggested that infectious complications (ICs) are clinically important [15]. The presence of an IC was defined by positive culture results. Since it is generally difficult to determine when one infection has resolved, and a second, independent infection has begun, the proportion of individual patients with positive cultures was abstracted in preference to the total number of positive cultures [31].
Statistics
Meta-analyses were conducted with a fixed effects model [32] using the odds ratio metric [33]. The presence of heterogeneity was assessed using the χ2 statistic [32] and the I2 measure [34]. The presence of a priori hypothesised subgroup differences were investigated using a formal test of treatment-subgroup interaction [35].
Primary analysis was conducted using Revman (RevMan version 4.2 for Windows, Oxford, UK; Cochrane Collaboration, 2003). Formal tests of treatment-subgroup interactions were conducted using PC SAS proc logist (version 6.12, SAS Institute, Cary, N.C. USA.). Primary stratification by study was maintained using dummy variable coding. A p value less than 0.05 indicated statistical significance, while a p value greater than 0.05 but less than 0.10 indicated a trend towards statistical significance.
A p value less than 0.10 was used to indicate the presence of potentially important heterogeneity or subgroup-treatment interactions.
Results
Literature search and study selection
Cross-searching Medline (from 1966) and EMBASE (from 1980) revealed 2, 287 unique abstracts. Independent review of all abstracts resulted in the retrieval of 465 papers. Figure 1 presents the results of the study selection process using the flow-diagram recommended by the Quality of Reporting of Meta-analyses (QUOROM) conference participants [36]. No non-English language publications were identified on this topic. Twenty-two trials were found to compare the effects of EN to PN on clinically meaningful outcomes in a critically ill patient population.
Five of the 22 publications were based on subgroups of patients that were reported in a subsequent larger published trial [37, 38, 39, 40, 41] and thus did not qualify for inclusion as individual trials.
Three of the remaining 17 trials compared immune enhanced EN and/or PN [42, 43, 44], and one trial was pseudo-randomised, using an alternating date of admission allocation sequence [45].
Of the remaining 13 trials one failed to report outcomes on 21% of all randomised patients [46], and a second failed to report outcomes on 12% of all randomised patients [47]. Neither of these trials reported loss to follow-up by study arm.
Nine studies qualified for consideration in the primary analysis with two additional studies qualifying for the sensitivity analysis. Eight studies presented 100% follow-up [48, 49, 50, 51, 52, 53, 54, 55], and one study reported complete follow-up (outcomes reported on 95% of randomised patients) [56]. One paper reported outcomes on 94.3% of randomised patients [57], and one reported outcomes on 91.5% of randomised patients [58]. Loss to follow-up in all three trials was reported by study arm.
Table 1 presents further details describing the study population, criteria used to identify the critically ill patient population and nutritional support goals for each of the 11 included trials.
True methodological quality
None of the 11 trials explicitly reported the maintenance of allocation concealment, and only one reported the use of blinding [48]. Nine of the 11 presented sufficient follow-up to allow the conduct of an ITT analysis.
Reporting of infectious complications
Six of the trials with complete follow-up reported positive cultures. Three trials reported the number of individual patients with positive cultures [48, 53, 56], and three reported the total number of positive cultures but not the number of patients with positive cultures [49, 55, 52]. The types of ICs reported by each trial are presented in Table 2.
Publication bias
Formal assessment of the funnel plot did not yield any evidence of a publication bias.
Primary analysis: mortality
Landmark mortality was the only clinically meaningful outcome reported in all trials. When the nine trials presenting ITT results were aggregated, a statistically significant mortality benefit was evident for the use of PN [odds ratio (OR) 0.51, 95% confidence interval (CI) 0.27–0.97, p=0.04; Fig. 2). The χ2 test for heterogeneity was non-significant (p=0.50) and the I2 measure was zero.
Primary analysis: infectious complications
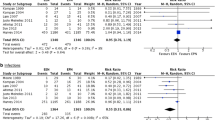
When the six trials reporting positive culture results were aggregated, there was a significant increase in ICs with PN use (OR 1.66, 95% CI 1.09–2.51; p=0.02; Fig. 3). The χ2 for heterogeneity was non-significant (p=0.16). The I2 measure of heterogeneity was 37.7%.
A priori subgroup analysis
Timing of feeding and effect on clinically meaningful outcomes
Six of the nine trials commenced enteral feeding within 24 h of intensive care admission or injury [48, 49, 53, 50, 51, 55]. In all cases early EN was achieved via transpyloric or jejunal feeding tubes.
Three trials did not meet the definition of early enteral feeding (<24 h). Two began EN within 48 h of ICU admission or injury [54, 56] while the third enrolled patients when there was an “actual or anticipated inadequate oral intake for longer than 7 days” [52].
Maintaining stratification by study, there was a potentially important (OR 1.07 PN vs. early EN, OR 0.29 PN vs. delayed EN, p=0.055) difference in the magnitude of the treatment effect for PN vs. early EN compared to PN vs. late EN (i.e. a treatment-subgroup interaction).
When the use of PN was compared to the provision of early EN, there was no significant effect on mortality (OR 1.07, 95% CI 0.39–2.95, p=0.89; Fig. 4). The χ2 test for statistical heterogeneity was non-significant (p=0.75) and the I2 measure was zero.
Compared to the provision of delayed EN there was a statistically significant mortality benefit in favour of the use of PN (OR 0.29, 95% CI 0.12–0.70, p=0.006). There was no evidence of statistical heterogeneity (p=0.60) and I2 was zero. Details regarding the timing of the onset of feeding for each trial are presented in Table 1.
Timing of feeding and effect on infectious complications
Of the six trials reporting ICs four compared early EN to PN. When aggregated, the use of PN approached a statistical trend towards more ICs compared to early EN (OR 1.47, 95% CI 0.90–2.38, p=0.12). Potentially important heterogeneity was present (p=0.07), and the I2measure was 56.9%. Only two trials comparing late EN to PN reported ICs [52, 56]. Due to the small number of trials, a meta-analysis could not be conducted. Individually neither trial reported a significant difference in ICs.
Sensitivity analysis
Two additional trials qualified for consideration in the sensitivity analysis [58]. Based on aggregation of all 11 trials the statistically significant mortality benefit remained in favour of PN use (OR 0.56, 95% CI 0.33–0.93, p=0.03; Fig. 4). The χ2 test for heterogeneity was non-significant (p=0.51) and the I2 was zero.
Both additional trials compared PN with late EN. In one study patients were required to have persistent coma (Glasgow Coma Scale <9) for at least 24 h prior to randomisation [58], and in the second patients were enrolled within 4–6 days of sepsis or surgery [57].
Considering these additional trials in the analysis of PN compared to late EN, the significant mortality benefit of PN remained (OR 0.44, 95% CI 0.24–0.81, p=0.008; Fig. 4). The test for statistical heterogeneity was non-significant (p=0.35) and the I2 measure was 10.0%.
Discussion
We used a component approach to assess the effect of methodological quality on the results obtained when trials comparing the use of standard PN to standard EN were aggregated. Since the extensive search did not detect any non-English language publications, it is unlikely a significant language bias exists [14] on this topic.
When ITT trials were considered, a statistically significant mortality benefit was evident in favour of PN (OR 0.51). Based on an a priori specified subgroup analysis this overall benefit was attributable to trials that compared the use of PN to delayed EN (OR 0.29). Although ICs were significantly increased (OR 1.66), given the evidence of a mortality benefit the clinical importance of these infectious complications should be questioned.
Clinically meaningful outcomes
Although the results of the current overview may appear novel, they are robust. When two additional trials were considered in the a priori defined sensitivity analysis, the statistically significant mortality benefit in favour of PN remained (OR 0.56), and, as suggested during the review process, when a more conservative random effects model was applied to the ITT trials, the benefit in favour of PN also remained (OR 0.49, 95% CI 0.25–0.98, p=0.04). Finally, although a previous review conducted on this topic reported non-significant results (OR 0.91, 95% CI 0.51–1.62), which resulted in a recommendation for the use of EN in preference to PN, it did not rule out the possibility of a mortality benefit in favour of PN [15]. Indeed, the point estimate of mortality benefit obtained in this current meta-analysis (OR 0.51) falls within the 95% confidence interval obtained in the previous review [15], which included numerous studies of questionable methodological quality [45, 46, 47].
The purpose of using a component approach to appraise trials was to investigate the impact of bias on the overall conclusions [14]. Nine of 11 trials considered in this meta-analysis presented complete (>95%) reporting and follow-up, which enables the conduct of an ITT analysis. An ITT analysis is known to provide the most conservative estimate of treatment effect [59] and protects against attrition bias, which results from non-random loss to follow-up [27]. Recent editorials reveal a strong prejudice against the use of PN in critically ill patients [60]. It is possible that the current findings are due to the exclusion of low-quality trials that overestimated the benefit of EN due to an inherent prejudice against PN use.
The results obtained from this current overview are also consistent with the evidence-based recommendations (EBRs) made in a recent cluster randomised trial evaluating the impact of evidence-based ICU feeding guidelines [7]. The guideline evaluated in this cluster randomised trial included a strong EBR for early feeding (within 24 h of ICU admission) with preference to the enteral route. If it was anticipated that enteral feeding could not be initiated within 24 h of ICU admission, the early use of PN was recommended. Our a priori specified subgroup analysis compared the effect of PN in trials that initiated early EN (<24 h) to the effect of PN in trials in which EN was delayed. While there was no benefit from the use of PN when EN was initiated early, there was a statistically significant benefit from the use of PN in trials in which EN was delayed (OR 0.29, p=0.006). The statistically significant benefit in this subgroup remained when additional trials were considered in the sensitivity analysis.
Infectious complications
The current overview documented a statistically significant increase in ICs associated with PN use (OR 1.66). A previous review also documented an increase in ICs (OR 2.51, 95% CI 1.66–3.79, p<0.0001) [15]. Although the point estimate for increased ICs obtained from the current overview (OR 1.66) is more conservative, it is contained within the 95% CI generated by the previous review, which included trials of questionable methodological quality [46, 47]. It is known that estimates of treatment effect decrease when more rigorous definitions of clinical infections are employed [61]. Previous reviews pooled suspected infections (e.g. fever of unknown origin) with positive culture results to obtain ‘total infectious complications’. Considering that no trials of PN vs. EN adjudicated suspected infections using an objective, blinded, repeatable process, it is likely that this outcome is highly susceptible to bias. We employed a more objective definition of infection based on positive culture results and obtained a marginally lower estimate of IC rates due to PN use.
A comprehensive review of the clinical importance of ICU-based infections found that the nature of the organism, the site of infection and the interaction between organism and site all had a significant impact on outcome [62]. Based on this finding an approach to classifying and combining ICU infections that accurately reflects the contribution that the infecting organism makes to patient outcome was proposed [62]. Due to incomplete reporting, it was not possible to classify and combine infections based on risk of outcome (e.g. severe infection, moderate infection, sub-clinical infection). Although the χ2 test for statistical heterogeneity was non-significant (p=0.16), the I2 measure of heterogeneity was 37.7%, suggesting that it may not be appropriate to pool all types of infections.
Statistical heterogeneity is said to be present in a meta-analysis when the differences in outcomes between studies is greater than expected by chance alone [34]. In the presence of unexplained heterogeneity pooling of study results may not be appropriate, even with a random effects model [32]. Although the χ2 test is commonly used to detect the presence of heterogeneity, this test has very poor power when the number of included studies is low [63]. The I2 statistic is an accepted measure of heterogeneity that does not depend on the number of included studies for interpretation [34]. Interpretation of the I2 measure reveals that 37.7% of the total variability in ICs was attributable to true differences between studies (heterogeneity) rather than random variability (sampling error). Although there are no clear guidelines as to what constitutes an unacceptable level of heterogeneity as measured by I2, a value of 20% reflects the presence of moderate heterogeneity [34].
Regardless of whether the clinician interprets information obtained from all trials (OR 1.66), from trials reporting the number of patients with infections (OR 1.88) or from trials reporting only the total number of infections (OR 1.46; Fig. 3), the ORs, and thus the estimate of increased ICs attributable to PN, are remarkably similar. Indeed, as suggested during the review process, if the incidence of infection is considered, which accounts for patient-time at risk, the OR does not change (OR 1.51). Regardless of the approach used to aggregate infections the clinician should be aware that the use of PN was associated with an increase in infectious complications. Because all infections do not lead to similar outcomes, and mutually inconsistent definitions of infections were used in each trial, the clinical importance of this finding is open to interpretation.
True methodological quality
Although previous reviewers have based their assessment of methodological quality on published manuscripts [15, 16, 17, 25, 64], it has been suggested that direct communication with the authors of contributing trials could improve our understanding of methodological quality. Due to resource and logistical constraints we were unable to communicate directly with the authors of the publications reviewed in this meta-analysis. Although the current state of knowledge regarding the importance of methodological quality is based on assessments of published manuscripts, not direct communication with authors [10, 12, 14, 22, 36], we believe that this is an area that merits further research.
The two main areas of methodological quality that were consistently found to be deficient in the manuscripts reviewed for this meta-analysis were the appropriate use of blinding and explicit reporting of allocation concealment.
Blinding
Since the appropriate use of blinding can reduce overoptimistic estimates of treatment effects by up to 26% [10], many novel and innovative processes have been developed for blinding complex intensive care interventions [65, 66]. Regardless of the complexity of the intervention, if a subjective outcome (e.g. ventilator-associated pneumonia, suspected infection) is important, it is always possible to blind outcome adjudicators. Because it may be important to understand whether patients, health care providers, researchers, outcome adjudicators, data collectors and even the data analysts were blinded, use of the term ‘double blinded’ is discouraged in preference to an explicit list of exactly who was blinded [67].
Allocation concealment
Trials with inadequate or unclear reporting of allocation concealment are known to produce up to 40% larger estimates of treatment effects [10, 12]. Allocation concealment refers to a process used to randomise participants so that patients, clinicians and researchers cannot predict or influence which participants are assigned to a given intervention (definition of allocation concealment contained on the Consolidated Standards of Reporting Trials web site http://www.consort-statement.org/allocationconcealment.htm, accessed 21 June 2004). A single sentence describing the use of ‘sealed, opaque, sequentially numbered envelopes’ or a ‘central call-in randomisation centre’ is sufficient to ensure the reader that allocation concealment was maintained [12].
Summary
The inclusion of trials of low methodological quality is known to have a substantial impact on the results and conclusions obtained from meta-analyses [14]. Based on the results of the nine trials presenting complete follow-up, meta-analysis revealed a significant mortality benefit in favour of the use of PN. A priori specified subgroup analysis demonstrated the benefit from PN use was greatest in trials in which EN was delayed (>24 h). Although we also documented an increase in ICs with PN use, because we were unable to separate sub-clinical infections from serious infections in the face of improved mortality the clinical importance of these ICs could not be established.
The overall findings of this meta-analysis would not lead us to recommend the use of PN in patients in whom EN could be initiated within 24 h of ICU admission or injury. In consideration of the overall results, including the increase in ICs, a grade B+ EBR [68] could be generated for the use of PN in patients in whom EN could not be initiated within 24 h of ICU admission or injury. This grade B+ EBR, where the ‘B’ indicates the recommendation is based on level II evidence and the ‘+’ indicates there is no heterogeneity between trials (‘Evidence-based Recommendations section of the Evidence-based Decision Making in Critical Care web site’ http://www.EvidenceBased.net, accessed 16 June 2004), is consistent with the explicit recommendation made for PN use in a recent cluster randomised trial of evidence-based ICU feeding guidelines that resulted in an overall 10% reduction in mortality [7].
It is important to recognise that the clinical trials included in this meta-analysis constitute level II evidence [68]. Because meta-analyses based on level II evidence often disagree with the findings of subsequent, well-conducted level I trials [8], we strongly recommend the conduct of a level I study addressing the use of standard PN in patients in whom standard EN cannot be started for at least 24 h after ICU admission or initial injury. This study should be adequately powered to detect a difference in a clinically meaningful outcome, should employ a blinded assessment of clinically important infections and should embrace all major aspects of study design that are known to reduce bias. In addition, the conduct of such a trial would present the ideal opportunity to conduct a full economic analysis of the use of PN compared to EN, expressed as costs per life saved [69].
References
Dudrick SJ, Wilmore DW, Vars HM, Rhoads JE (1968) Long-term total parenteral nutrition with growth, development, and positive nitrogen balance. Surgery 64:134–142
Dudrick SJ (2003) Early developments and clinical applications of total parenteral nutrition. JPEN J Parenter Enteral Nutr 27:291–299
Spencer CTCC (2001) The development of TPN: an interview with pioneer surgical nutritionist Jonathan E. Rhoads, MD. J Am Diet Assoc 101:747–750
Varga P, Griffiths R, Chiolero R, Nitenberg G, Leverve X, Pertkiewicz M, Roth E, Wernerman J, Pichard C, Preiser JC (2003) Is parenteral nutrition guilty? Intensive Care Med 29:1861–1864
Marik PE, Pinsky M (2003) Death by parenteral nutrition. Intensive Care Med 29:2104
Preiser JC, Berre J, Carpentier Y, Jolliet P, Pichard C, Van Gossum A, Vincent JL (1999) Management of nutrition in European intensive care units: results of a questionnaire. Working Group on Metabolism and Nutrition of the European Society of Intensive Care Medicine. Intensive Care Med 25:95–101
Martin CM, Doig GS, Heyland DK, Morrison T, Sibbald WJ (2004) Multicentre, cluster-randomized clinical trial of algorithms for critical-care enteral and parenteral therapy (ACCEPT). Can Med Assoc J 170:197–204
LeLorier J, Gregoire G, Benhaddad A, Lapierre J, Derderian F (1997) Discrepancies between meta-analyses and subsequent large randomized, controlled trials. N Engl J Med 337:536–542
Smith R, Chalmers I (2001) Britain’s gift: a “Medline” of synthesised evidence. BMJ 323:1437–1438
Juni P, Altman DG, Egger M (2001) Systematic reviews in health care: assessing the quality of controlled clinical trials. BMJ 323:42–46
Cochrane Injuries Group Albumin Reviewers (1998) Human albumin administration in critically ill patients: systematic review of randomised controlled trials. BMJ 317:235–240
Schulz KF, Grimes DA (2002) Allocation concealment in randomised trials: defending against deciphering. Lancet 359:614–618
Wilkes MM, Navickis RJ (2001) Patient survival after human albumin administration. A meta-analysis of randomized, controlled trials. Ann Intern Med 135:149–164
Egger M, Juni P, Bartlett C, Holenstein F, Sterne J (2003) How important are comprehensive literature searches and the assessment of trial quality in systematic reviews? Empirical study. Health Technol Assess 7:1–76
Heyland DK, Dhaliwal R, Drover JW, Gramlich L, Dodek P (2003) Canadian clinical practice guidelines for nutrition support in mechanically ventilated, critically ill adult patients. JPEN J Parenter Enteral Nutr 27:355–373
Heyland DK, Novak F, Drover JW, Jain M, Su X, Suchner U (2001) Should immunonutrition become routine in critically ill patients? A systematic review of the evidence. JAMA 286:944–953
Heyland DK (2000) Enteral and parenteral nutrition in the seriously ill, hospitalized patient: a critical review of the evidence. J Nutr Health Aging 4:31–41
Heyland DK, MacDonald S, Keefe L, Drover JW (1998) Total parenteral nutrition in the critically ill patient: a meta-analysis. JAMA 280:2013–2019
Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJ, Gavaghan DJ, McQuay HJ (1996) Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials 17:1–12
Moher D, Jadad AR, Nichol G, Penman M, Tugwell P, Walsh S (1995) Assessing the quality of randomized controlled trials: an annotated bibliography of scales and checklists. Control Clin Trials 16:62–73
Juni P, Witschi A, Bloch R, Egger M (1999) The hazards of scoring the quality of clinical trials for meta-analysis. JAMA 282:1054–1060
Huwiler-Muntener K, Juni P, Junker C, Egger M (2002) Quality of reporting of randomized trials as a measure of methodologic quality. JAMA 287:2801–2804
Haynes R, Wilczynski N, McKibbon A, Walker C, Sinclair J (1994) Developing Optimal Search Strategies for Detecting Clinically Sound Studies in MEDLINE. J Am Med Inform Assoc 1:447–458
Juni P, Holenstein F, Sterne J, Bartlett C, Egger M (2002) Direction and impact of language bias in meta-analyses of controlled trials: empirical study. Int J Epidemiol 31:115–123
Doig GS, Simpson F, Delaney A (2004) A review of the true methodological quality of nutritional support trials conducted in the critically ill: time for improvement. Anesth Analg (in press)
Graf J, Doig GS, Cook DJ, Vincent JL, Sibbald WJ (2002) Randomized, controlled clinical trials in sepsis: has methodological quality improved over time? Crit Care Med 30:461–472
Lachin JM (2000) Statistical considerations in the intent-to-treat principle. Control Clin Trials 21:167–189
Flather MD, Farkouth ME, Pogue JM, Yusuf S (1997) Strengths and limitations of meta-analysis: larger studies may be more reliable. Control Clin Trials 18:568–579
Prentice RL (1989) Surrogate endpoints in clinical trials: definition and operational criteria. Stat Med 8:431–440
Novak F, Heyland DK, Avenell A, Drover JW, Su X (2002) Glutamine supplementation in serious illness: a systematic review of the evidence. Crit Care Med 30:2022–2029
Kleinbaum DG, Kupper LL, Morgenstern H (1982) Measures of disease frequency: incidence. In: Kleinbaum DG, Kupper LL, Morgenstern H (eds) Epidemiological research: principles and quantitative methods. Van Nostrand Reinhold, New York, pp 96–115
Villar J, Mackey ME, Carroli G, Donner A (2001) Meta-analyses in systematic reviews of randomized controlled trials in perinatal medicine: comparison of fixed and random effects models. Stat Med 20:3635–3647
Deeks JJ (2002) Issues in the selection of a summary statistic for meta-analysis of clinical trials with binary outcomes. Stat Med 21:1575–1600
Higgins JP, Thompson SG (2002) Quantifying heterogeneity in a meta-analysis. Stat Med 21:1539–1558
Brookes ST, Whitley E, Peters TJ, Mulheran PA, Egger M, Davey SG (2001) Subgroup analyses in randomised controlled trials: quantifying the risks of false-positives and false-negatives. Health Technol Assess 5:1–56
Moher D, Cook DJ, Eastwood S, Olkin I, Rennie D, Stroup DF (1999) Improving the quality of reports of meta-analyses of randomised controlled trials: the QUOROM statement. Quality of Reporting of Meta-analyses. Lancet 354:1896–1900
Braga M, Vignali A, Gianotti L, Cestari A, Profili M, Di C, V (1995) Benefits of early postoperative enteral feeding in cancer patients. Infusionsther Transfusionsmed 22:280–284
Gianotti L, Braga M, Gentilini O, Balzano G, Zerbi A, Di Carlo V (2000) Artificial nutrition after pancreaticoduodenectomy. Pancreas 21:344–351
Di Carlo, V, Gianotti L, Balzano G, Zerbi A, Braga M (1999) Complications of pancreatic surgery and the role of perioperative nutrition. Dig Surgery 16:320–326
Braga M, Gianotti L, Vignali A, Cestari A, Bisagni P, Di C, V (1998) Artificial nutrition after major abdominal surgery: impact of route of administration and composition of the diet. Crit Care Med 26:24–30
Braga M, Vignali A, Gianotti L, Cestari A, Profili M, Di C, V (1996) Immune and nutritional effects of early enteral nutrition after major abdominal operations. Eur J Surgery 162:105–112
Bertolini G, Iapichino G, Radrizzani D, Facchini R, Simini B, Bruzzone P, Zanforlin G, Tognoni G (2003) Early enteral immunonutrition in patients with severe sepsis: results of an interim analysis of a randomized multicentre clinical trial. Intensive Care Med 29:834–840
Hadfield RJ, Sinclair DG, Houldsworth PE, Evans TW (1995) Effects of enteral and parenteral nutrition on gut mucosal permeability in the critically ill. Am J Respir Crit Care Med 152:1545–1548
Wischmeyer PE, Lynch J, Liedel J, Wolfson R, Riehm J, Gottlieb L, Kahana M (2001) Glutamine administration reduces Gram-negative bacteremia in severely burned patients: a prospective, randomized, double-blind trial versus isonitrogenous control. Crit Care Med 29:2075–2080
Hadley MN, Grahm TW, Harrington T, Schiller WR, McDermott MK, Posillico DB (1986) Nutritional support and neurotrauma: a critical review of early nutrition in forty-five acute head injury patients. Neurosurgery 19:367–373
Moore FA, Moore EE, Jones TN, McCroskey BL, Peterson VM (1989) TEN versus TPN following major abdominal trauma-reduced septic morbidity. J Trauma 29:916–922
Young B, Ott L, Twyman D, Norton J, Rapp R, Tibbs P, Haack D, Brivins B, Dempsey R (1987) The effect of nutritional support on outcome from severe head injury. J Neurosurg 67:668–676
Rayes N, Hansen S, Seehofer D, ller AR, Serke S, Bengmark S, Neuhaus P (2002) Early enteral supply of fiber and Lactobacilli versus conventional nutrition: a controlled trial in patients with major abdominal surgery. Nutrition 18:609–615
Reynolds JV, Kanwar S, Welsh FKS, Windsor ACJ, Murchan P, Barclay GR, Guillou PJ (1997) Does the route of feeding modify gut barrier function and clinical outcome in patients after major upper gastrointestinal surgery? JPEN J Parenter Enteral Nutr 21:196–201
Kudsk KA, Minard G, Wojtysiak SL, Croce M, Fabian T, Brown RO (1994) Visceral protein response to enteral versus parenteral nutrition and sepsis in patients with trauma. Surgery 116:516–523
Dunham CM, Frankenfield D, Belzberg H, Wiles C, Cushing B, Grant Z (1994) Gut failure-predictor of or contributor to mortality in mechanically ventilated blunt trauma patients? J Trauma 37:30–34
Woodcock NP, Zeigler D, Palmer MD, Buckley P, Mitchell CJ, MacFie J (2001) Enteral versus parenteral nutrition: a pragmatic study. Nutrition 17:1–12
Gianotti L, Braga M, Vignali A, Balzano G, Zerbi A, Bisagni P, Di C, V, Fischer JE, Rombeau J, Harrington D (1997) Effect of route of delivery and formulation of postoperative nutritional support in patients undergoing major operations for malignant neoplasms. Arch Surg 132:1222–1230
Rapp RP, Young B, Twyman D, Bivins BA, Haack D, Tibbs PA, Bean JR (1983) The favorable effect of early parenteral feeding on survival in head-injured patients. J Neurosurg 58:906–912
Adams S, Dellinger EP, Wertz MJ, Oreskovich MR, Simonowitz D, Johansen K (1986) Enteral versus parenteral nutritional support following laparotomy for trauma: a randomized prospective trial. J Trauma 26:882–891
Kalfarentzos F, Kehagias J, Mead N, Kokkinis K, Gogos CA (1997) Enteral nutrition is superior to parenteral nutrition in severe acute pancreatitis: results of a randomized prospective trial. Br J Surg 84:1665–1669
Cerra FB, McPherson JP, Konstantinides FN, Konstantinides NN, Teasley KM (1988) Enteral nutrition does not prevent multiple organ failure syndrome (MOFS) after sepsis. Surgery 104:727–733
Borzotta AP, Pennings J, Papasadero B, Paxton J, Mardesic S, Borzotta R, Parrott A, Bledsoe F (1994) Enteral versus parenteral nutrition after severe closed head injury. J Trauma 37:459–468
Hollis S, Campbell F (1999) What is meant by intention to treat analysis? Survey of published randomised controlled trials. BMJ 319:670–674
Heyland DK (2000) Parenteral nutrition in the critically-ill patient: more harm than good? Proc Nutr Soc 59:457–466
van Nieuwenhoven CA, Buskens E, van Tiel FH, Bonten MJ (2001) Relationship between methodological trial quality and the effects of selective digestive decontamination on pneumonia and mortality in critically ill patients. JAMA 286:335–340
Cohen J, Cristofaro P, Carlet J, Opal S (2004) A new method of classifying infections in critically ill patients. Crit Care Med 32:1510–1534
Hardy RJ, Thompson SG (1998) Detecting and describing heterogeneity in meta-analysis. Stat Med 17:841–856
Preiser JC, Chiolero R, Wernerman J (2003) Nutritional papers in ICU patients: what lies between the lines? Intensive Care Med 29:156–166
Finfer S, Bellomo R, Myburgh J, Norton R (2003) Efficacy of albumin in critically ill patients. BMJ 326:559–560
Dellinger RP, Zimmerman JL, Taylor RW, Straube RC, Hauser DL, Criner GJ, Davis K Jr, Hyers TM, Papadakos P (1998) Effects of inhaled nitric oxide in patients with acute respiratory distress syndrome: results of a randomized phase II trial. Inhaled Nitric Oxide in ARDS Study Group. Crit Care Med 26:15–23
Schulz KF, Chalmers I, Altman DG (2002) The landscape and lexicon of blinding in randomized trials. Ann Intern Med 136:254–259
Cook DJ (1995) Clinical trials in the treatment of sepsis: an evidence-based approach. In: Sibbald WJ, Vincent JL (eds) Clinical trials for the treatment of sepsis. Springer, Berlin Heidelberg New York, pp XIX–XXXI
Drummond MF, Stoddart GL, Torrance GW (1995) Cost-effectiveness analysis In: Drummond MF, Stoddart GL, Torrance GW (eds) Methods for the economic evaluation of health care programmes. Oxford University Press, New York, pp 74–112
Acknowledgements
This work was supported in part by grants from the Australian and New Zealand Intensive Care Foundation and the NorthCare Foundation.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Simpson, F., Doig, G.S. Parenteral vs. enteral nutrition in the critically ill patient: a meta-analysis of trials using the intention to treat principle. Intensive Care Med 31, 12–23 (2005). https://doi.org/10.1007/s00134-004-2511-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00134-004-2511-2