Abstract
Aims/hypothesis
Diabetogenic effects of per- and polyfluoroalkyl substances (PFAS) have been suggested. However, evidence based on prospective cohort studies is limited. We examined the association between serum PFAS concentrations and incident diabetes in the Study of Women’s Health Across the Nation Multi-Pollutant Study (SWAN-MPS).
Methods
We included 1237 diabetes-free women aged 45–56 years at baseline (1999–2000) who were followed up to 2017. At each follow-up visit, women with incident diabetes were identified by the presence of one or more of the following conditions: (1) use of a glucose-lowering medication at any visit; (2) fasting glucose ≥7 mmol/l on two consecutive visits while not on steroids; and (3) any two visits with self-reported diabetes and at least one visit with fasting blood glucose ≥7 mmol/l. Serum concentrations of 11 PFAS were quantified by online solid-phase extraction–HPLC–isotope dilution–tandem MS. Seven PFAS with high detection rates (>96%) (n-perfluorooctanoic acid [n-PFOA], perfluorononanoic acid [PFNA], perfluorohexane sulfonic acid [PFHxS], n-perfluorooctane sulfonic acid [n-PFOS], sum of perfluoromethylheptane sulfonic acid isomers [Sm-PFOS], 2-[N-methyl-perfluorooctane sulfonamido] acetic acid [MeFOSAA] and 2-[N-ethyl-perfluorooctane sulfonamido] acetic acid) were included in data analysis. Cox proportional hazards models were used to compute HRs and 95% CIs. Quantile-based g-computation was used to evaluate the joint effects of PFAS mixtures.
Results
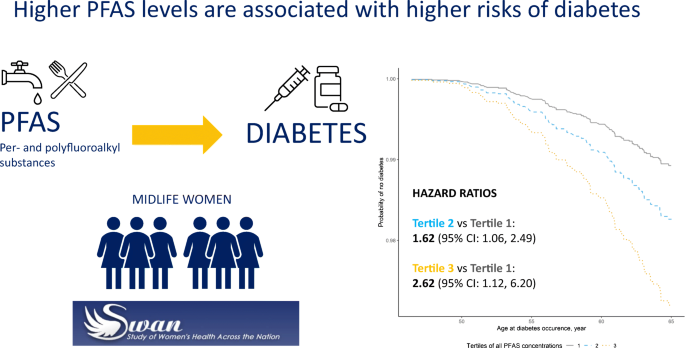
After adjustment for race/ethnicity, site, education, smoking status, alcohol consumption, total energy intake, physical activity, menopausal status and BMI, the HR (95% CI) comparing the lowest with the highest tertile was 1.67 (1.21, 2.31) for n-PFOA (ptrend = 0.001), 1.58 (1.13, 2.21) for PFHxS (ptrend = 0.003), 1.36 (0.97, 1.90) for Sm-PFOS (ptrend = 0.05), 1.85 (1.28, 2.67) for MeFOSAA (ptrend = 0.0004) and 1.64 (1.17, 2.31) for the sum of four common PFAS (n-PFOA, PFNA, PFHxS and total PFOS) (ptrend = 0.002). Exposure to seven PFAS as mixtures was associated with an HR of 2.62 (95% CI 1.12, 6.20), comparing the top with the bottom tertiles for all seven PFAS.
Conclusions/interpretation
This study suggests that PFAS may increase diabetes risk in midlife women. Reduced exposure to these ‘forever and everywhere chemicals’ may be an important preventative approach to lowering population-wide diabetes risk.
Graphical abstract
Similar content being viewed by others

Introduction
Per- and polyfluoroalkyl substances (PFAS) are ubiquitous, synthetic compounds used in industrial and consumer applications, such as non-stick cookware, food packaging and food contact materials, carpeting, apparels and firefighting foams [1, 2]. These chemicals, especially long-chain PFAS such as perfluorooctanoic acid (PFOA) and perfluorooctane sulfonic acid (PFOS), are of public health concern because they are very persistent in the environment and in the human body [1, 2]. Recently, these chemicals received attention because they were detected in drinking water, affecting more than 200 million residents in the USA [3]. PFAS have, therefore, been detected in the blood of almost all people tested in the USA [4, 5]. The text box summarises potential sources of human exposure to PFAS. A recent review suggests that PFOA, PFOS and other PFAS may be associated with pre-eclampsia, altered hepatic enzyme levels, increased serum lipids, decreased antibody response to vaccines and low birthweight, although their causal relationships need to be assessed [1].

PFAS have also been suggested as potential diabetogens [6]. The structure of PFAS (CnF2n+1-R) consists of perfluoroalkyl chain (CnF2n+1) with an attached functional group (R), either carboxylic (-COOH) or sulfonic acids (-SO3H) [7]. Figure 1 illustrates the structures of PFOA and PFOS. Their structures are similar to that of fatty acids, which contain a hydrocarbon chain and carboxylic acid group. PFAS, therefore, have chemical properties similar to those of fatty acids, well-known endogenous ligands of peroxisome proliferator-activated receptors (PPARs) [8]. Known as lipid and insulin sensors, PPARs are main regulators of adipocyte differentiation and lipid and glucose homeostasis [8]. A major physiological pathway through which PFAS may affect diabetes risk is interference with PPARs, resulting in activation or inhibition of PPARs [9]. Experimental studies suggest that PFAS at human-relevant exposure levels may promote adipocyte proliferation and differentiation through PPARα and/or PPARγ and play a role in inflammation, and lipid and glucose metabolism [10, 11]. Epidemiological evidence of the association between PFAS and type 2 diabetes is limited. Cross-sectional [12,13,14,15,16] and case–control studies with prevalent cases [17, 18] are subject to reverse causality given that reduced kidney function, a well-known complication of diabetes, may lead to accumulation of circulating PFAS due to decreased PFAS excretion [19, 20]. There are mixed findings in prospective studies, with two studies reporting positive associations of PFOS and/or PFOA with incident type 2 diabetes [21, 22], and others reporting no associations [23, 24]. More evidence from population-based prospective cohort studies is needed to determine causal links between PFAS exposures and diabetes.
The aim of the present study was to examine the association between serum PFAS concentrations and incident diabetes in the Study of Women’s Health Across the Nation (SWAN). We also evaluated the overall joint effect of PFAS mixtures to determine whether the overall joint effect is greater than the associations for individual PFAS [25, 26].
Methods
Study population
SWAN is an ongoing, multi-site, multi-ethnic, community-based prospective cohort study of midlife women to characterise the menopausal transition and its association with subsequent health endpoints (http://www.swanstudy.org) [27]. In 1996–1997, 3302 premenopausal women were recruited at seven study sites in the USA. White women were recruited at each site and women of other races/ethnicities were recruited as follows: Black women from Boston, MA, Pittsburgh, PA, southeast Michigan and Chicago, IL; Hispanic women from Newark, NJ; Chinese women from Oakland, CA; and Japanese women from Los Angeles, CA. Eligibility criteria for the SWAN included age 42–52 years, having an intact uterus, having at least one ovary, having at least one menstrual period, no use of hormone therapy in the past 3 months, and self-identifying with the designated racial/ethnic groups. Participants returned for regular clinical examinations approximately annually. The institutional review board at each participating site approved the study protocol, and all participants provided written informed consent at each study visit.
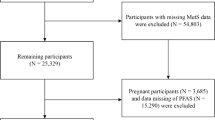
The SWAN-Multi-Pollutant Study (MPS) was initiated to evaluate the roles of multiple environmental pollutants in chronic diseases during and after the menopausal transition. Environmental chemical exposure assessment, including PFAS, was conducted using repository serum and urine samples from the third SWAN follow-up (MPS baseline, 1999–2000, n = 2694). The SWAN-MPS was designed to evaluate multiple environmental chemicals measured in serum and urine, one class at a time as well as all chemicals as mixtures; participants who had sufficient volumes of both serum and urine were eligible. Women from Chicago (n = 368) and Newark (n = 278) were not eligible because urine samples were not collected at these sites. Women with insufficient serum or urine samples were excluded (n = 648), resulting in a base sample of 1400 women from four racial/ethnic groups (White, Black, Chinese and Japanese) for the SWAN-MPS. Women excluded due to insufficient serum/urine samples, compared with those included in the SWAN-MPS, were more likely to be obese, former or current smokers, and physically inactive, and to have lower education levels (electronic supplementary material [ESM] Table 1). For the current analysis, we excluded 82 women with prevalent diabetes at SWAN-MPS baseline and 81 women who had missing data for the key covariates, yielding a final analytical sample of 1237 women followed from 1999/2000 through 2017 (ESM Fig. 1).
Incident diabetes
At each follow-up visit, women with incident diabetes were identified by the presence of one or more of the following conditions: (1) use of a glucose-lowering medication at any visit; (2) fasting glucose ≥7 mmol/l on two consecutive visits while not on steroids; and (3) any two visits with self-reported diabetes and at least one visit with fasting blood glucose ≥7 mmol/l. The date of the SWAN visit was used instead of the actual date of diagnosis. See ESM Methods for further details of incident diabetes ascertainment. Fasting serum glucose level was measured by the hexokinase method (Boehringer Mannheim Diagnostics, Indianapolis, IN, USA). The type of diabetes (i.e. type 1 or type 2) was not determined but most of the incident cases of diabetes at this life stage can be assumed to be type 2.
Serum PFAS concentrations
Serum samples collected at SWAN-MPS baseline were sent to the Division of Laboratory Sciences, National Center for Environmental Health, Centers for Disease Control and Prevention (CDC) for PFAS concentration assessments. The laboratory’s involvement did not constitute engagement in human studies. Online solid-phase extraction–HPLC–isotope dilution–tandem MS (SPE-HPLC-MS/MS) was used to quantify concentrations of 11 PFAS: linear PFOA (n-PFOA); sum of branched PFOA isomers (Sb-PFOA); perfluorononanoic acid (PFNA); perfluorodecanoic acid (PFDA); perfluoroundecanoic acid (PFUnDA); perfluorododecanoic acid (PFDoDA); perfluorohexane sulfonic acid (PFHxS); linear PFOS (n-PFOS); sum of perfluoromethylheptane sulfonic acid isomers (Sm-PFOS); 2-(N-methyl-perfluorooctane sulfonamido) acetic acid (MeFOSAA); and 2-(N-ethyl-perfluorooctane sulfonamido) acetic acid (EtFOSAA) [28]. Total PFOS concentration was computed as the sum of linear and branched PFOS. The analytic methods and quality control procedures have been described previously [29, 30]. The limit of detection (LOD) for all analytes was 0.1 ng/ml. We did not include Sb-PFOA, PFDA, PFUnDA or PFDoDA in data analyses due to low detection rates (<50%). Detection rates of all included PFAS were >96% (ESM Table 2). We also computed the sum of n-PFOA, PFNA, PFHxS and total PFOS because the recent risk assessment of the European Food Safety Authority (EFSA) Panel on Contaminants in the Food Chain was based on the sum of these four common PFAS [31]. Participants with PFAS concentrations below LODs were assigned values equal to LOD/√2. The CV of low- and high-concentration quality controls ranged from 6% to 12%, depending on the analyte.
Other covariates
Sociodemographic variables including age, race/ethnicity (White, Black, Chinese, Japanese) and educational attainment (≤high school, some college, ≥college degree) were ascertained through a self-administered questionnaire at baseline. At each follow-up visit, cigarette smoking (never, former, current smoker), alcohol consumption (<1 drink/month, ≥1 drink/month and <2 drinks/week, ≥2 drinks/week), physical activity, total energy intake, parity (nulliparous, 1 or 2 births, 3+ births) and menopausal status (premenopausal, postmenopausal, unknown due to hormone therapy) were obtained from standardised interviews. Physical activity (score from 3 to 15) was assessed using a modified Kaiser Physical Activity Survey to determine the physical activity level in active living, household/caregiving and sports/exercise during the past 12 months. Total energy intake was calculated by summing the production of each food’s consumption frequency from a detailed semi-quantitative food frequency questionnaire by the corresponding energy content. BMI was calculated as weight (kg) divided by squared height (m). We did not consider time-varying BMI and menopausal status given the evidence that these variables may be affected by PFAS and thus introduce overadjustment bias [32, 33].
Statistical analysis
Univariate statistics were calculated and compared by incident diabetes using χ2 test for categorical variables and Wilcoxon rank-sum test for continuous variables. Cox proportional hazards models were used to calculate HRs and 95% CIs for incident diabetes associated with each PFAS concentration. We used age as the time scale, and participants contributed survival time from the SWAN-MPS baseline to the date of the first diabetes diagnosis for incident cases or the last follow-up visit for non-cases. To capture potential non-linear associations as well as to ensure enough cases of diabetes in each exposure group, serum PFAS concentrations were categorised into tertiles. HRs (95% CIs) of diabetes were calculated comparing the highest/middle tertile with the lowest tertile of PFAS concentrations (reference group). A linear trend of the association across the tertiles was tested by including PFAS tertiles as a continuous variable. We also examined the associations using log2-transformed continuous PFAS concentrations. HRs were interpreted as effect per doubling of PFAS concentration. Potential confounders were chosen based on prior knowledge [34] and adjusted progressively. The base model adjusted for race/ethnicity and study sites. The full model additionally adjusted for education, smoking status, alcohol consumption, physical activity score, total energy intake, menopausal status (baseline) and BMI (baseline). Smoking status, alcohol consumption, physical activity and total energy intake were time-varying. The proportional hazards assumption was checked using the scaled Schoenfeld residuals, and no significant violations were found. As sensitivity analyses, we evaluated the impact of additional adjustment for meat consumption and parity as well as time-on-study (time since baseline) with adjustment for age as a covariate instead of age as the time scale. We also computed race-specific associations by including interactions between each PFAS and either Black or Asian women compared with White women.
We recognised that selective participation in the SWAN-MPS might have biased the associations between PFAS and incident diabetes. We expected that estimates of the PFAS–diabetes association would be weaker than the true causal effects because women who might be more susceptible to metabolic effects of PFAS were more likely to have dropped out or developed diabetes before the baseline of the SWAN-MPS. Women excluded due to insufficient biospecimens were less educated and had higher BMI (ESM Table 1); these characteristics are related to high PFAS exposure [29] and higher risk of diabetes [34]. Therefore, restriction to those who participated in the SWAN-MPS could lead to underestimation. To reduce this potential bias, we used inverse probability weighting (IPW) to develop a non-response weight to create a pseudo population representing the women who were at risk of incident diabetes at the time of serum PFAS measurements in the original SWAN cohort. See ESM Methods for further details illustrating the construction of IPW.
We used the quantile-based g-computation to investigate the joint effects of PFAS mixtures on incident diabetes [35]. Serum concentrations of all seven PFAS were included in the same quantile-based g-computation model, and the joint effect was evaluated through estimating the HR of diabetes for a one quantile simultaneous increase in all PFAS in this specified mixture. For the current analysis, the quantile-based g-computation was implemented by categorising all PFAS into tertiles. Each PFAS component was assigned a weight corresponding to the proportion of the joint effect of the mixture attributed to that component. When coefficients of PFAS components were not in the same direction, two sets of weights that yielded the proportion of the positive (or negative) ‘partial effect’ of all PFAS components with positive (or negative) coefficients were calculated (ESM Fig. 2). The same covariates used in the full models above were used in the quantile-based g-computation model. The R package ‘qgcomp’ (version 2.8.5) was used [35].
All statistical analyses were conducted using SAS (version 9.4 SAS Institute, Cary, NC, USA) and R (version 4.0.5; R Foundation for Statistical Computing). Statistical significance was defined at α <0.05.
Results
At SWAN-MPS baseline, the median age was 49.4 years (IQR 47.4–51.5) and the proportions of each racial/ethnic group were 51.7% for White, 19.2% for Black, 13.3% for Chinese, and 15.8% for Japanese (Table 1). Among 1237 participants at risk for diabetes at SWAN-MPS baseline, 102 developed incident diabetes during 17,005 person-years of follow-up, an incidence of 6 per 1000 person-years. Compared with women who remained free of diabetes throughout follow-up, those who developed diabetes were more likely to be Black, from southeast Michigan, less educated and less physically active, to have a higher energy consumption and to have higher BMI at baseline. Women with incident diabetes had higher serum concentrations of total PFOS, n-PFOS, Sm-PFOS, MeFOSAA and EtFOSAA than those without incident diabetes. Women with higher concentrations of the sum of n-PFOA, PFNA, PFHxS and total PFOS were more likely to be Black, from southeast Michigan, former or current smokers and postmenopausal, and to have higher BMI (ESM Table 3).
Associations between serum PFAS concentrations and incident diabetes are presented in Table 2. After adjusting for race/ethnicity, study site, education, smoking status, alcohol consumption, physical activity score, total energy intake, menopausal status and BMI (full model), the HRs (95% CIs) for the highest vs lowest tertile were 1.67 (1.21, 2.31) for n-PFOA (ptrend = 0.001), 1.58 (1.13, 2.21) for PFHxS (ptrend = 0.003), 1.36 (0.97, 1.90) for Sm-PFOS (ptrend = 0.05), 1.85 (1.28, 2.67) for MeFOSAA (ptrend = 0.0004), and 1.64 (1.17, 2.31) for the sum of four common PFAS (n-PFOA, PFNA, PFHxS and total PFOS) (ptrend = 0.002). Statistically significant positive associations were also observed for total PFOS, n-PFOS and MeFOSAA when they were fit as continuous variables (log2-transformed). Fully adjusted HRs (95% CIs) for diabetes associated with each doubling of serum concentration were 1.22 (1.05, 1.43) for total PFOS, 1.22 (1.04, 1.42) for n-PFOS and 1.23 (1.07, 1.42) for MeFOSAA. The results remained essentially unchanged when time-on-study instead of age was used as the time scale except PFNA now reached statistical significance (HR 1.42 [95% CI 1.00, 2.00]) for highest vs lowest tertile (ptrend = 0.05) (ESM Table 4). Additional adjustment for meat consumption or parity did not change the results (ESM Table 5). As expected, the results were attenuated when the models did not account for potential selection bias (i.e. without IPW) (ESM Table 6). The associations between PFAS and diabetes were generally larger and statistically significant only among White women but no associations were found among Black or Asian women except for MeFOSAA among Black women (ESM Table 7).
In the quantile-based g-computation evaluating the overall joint effects of PFAS mixtures (Fig. 2), increasing all seven PFAS serum concentrations by one tertile was associated with an elevated incidence of diabetes (HR 1.62 [95% CI 1.06, 2.49]) in the fully adjusted model. The HR comparing tertile 3 vs tertile 1 for all seven PFAS concentrations was 2.62 (95% CI 1.12, 6.20).
Adjusted survival curves for incident diabetes by tertile of serum concentrations of PFAS mixtures from quantile-based g-computation, after adjusting for race/ethnicity, study site, education, smoking status, alcohol consumption, physical activity score, total energy intake, menopausal status (baseline level) and BMI (baseline level). HR 1.62 (95% CI 1.06, 2.49) for increasing all seven PFAS concentrations by one tertile. HR 2.62 (95% CI 1.12, 6.20) for comparing tertile 3 (dotted yellow line) vs tertile 1 (solid grey line) for all seven PFAS concentrations
Discussion
This prospective cohort study supports the hypothesis that higher serum concentrations of n-PFOA, PFHxS, PFOS and MeFOSAA are associated with higher risk of incident diabetes. Combined exposure to seven PFAS was associated with an HR of 2.62 (95% CI 1.12, 6.20) when comparing the top vs the bottom tertile for all seven PFAS; this risk was greater than that seen with individual PFAS (HR range 1.36–1.85), suggesting a potential additive or synergistic effect of multiple PFAS on diabetes risk.
The large magnitude of the association between combined exposure to PFAS and incident diabetes from the mixture analysis (quantile-based g-computation) also suggests that PFAS may have substantial clinical impacts on diabetes risk. The HR of 2.62 was roughly equivalent to the magnitude of the HR for overweight individuals (25 kg/m2 ≤ BMI <30 kg/m2) vs those with BMI <25 kg/m2 (HR 2.89) observed in our study (ESM Table 8) and the pooled RR (2.99) from a meta-analysis [36], and even greater than that for current smokers vs never smokers (HR 2.30) in our population and the pooled RR (1.57) from a meta-analysis [37]. Given the widespread exposure to PFAS in the general population, the expected benefit of reducing exposure to these ubiquitous chemicals might be considerable.
Our findings are in line with those of the Nurses’ Health Study-II (NHS-II) nested case–control study with 1586 women aged 45.3 ± 4.4 years (793 case–control pairs) [21]. They found that fully adjusted ORs (95% CIs) for incident diabetes comparing the top with the bottom tertile of plasma concentrations were 1.54 (1.04, 2.28) for PFOA and 1.62 (1.09, 2.41) for PFOS. The corresponding HRs (95% CIs) in our study were 1.67 (1.21, 2.31) for PFOA and 1.25 (0.90, 1.74) for PFOS. When log2-transformed PFAS were modelled, ORs (95% CIs) were 1.29 (1.07, 1.56) for PFOA and 1.20 (0.97, 1.47) for PFOS (recalculated using the reported ORs for a 1-SD increase in log10-transformed PFAS) in the NHS-II. The corresponding HRs (95% CIs) in our study were 1.10 (0.94, 1.29) for PFOA and 1.22 (1.05, 1.43) for PFOS. This suggests that the associations between the two studies are comparable depending on the shape of the dose–response associations. We observed a significant HR of 1.58 (95% CI 1.13, 2.21) (tertile 3 vs tertile 1) for PFHxS, whereas the NHS-II found a non-significant association (OR 1.28 [95% CI 0.86, 1.86]). PFNA was not significantly associated with incident diabetes in either study.
Another prospective study of 957 adults from the Diabetes Prevention Program (DPP) trial and Diabetes Prevention Program Outcomes Study (DPPOS) (aged ≥25 years, 65.3% female sex, PFAS concentrations from plasma collected at baseline [1996–1999] and the second year of follow-up) found that a doubling of branched PFOA was associated with an HR of 1.14 (95% CI 1.04, 1.25) in the placebo group but no association was observed in the lifestyle intervention group [22]. Other PFAS were not associated with incident diabetes in either group.
Two other prospective studies failed to detect significant associations between PFAS and incident diabetes [23, 24]. A large cohort study of 32,254 community residents and workers (aged 20–110 years; 53.8% female sex; annual retrospective PFOA serum concentrations estimated from 1951 or participant’s birth year through 2011) who were exposed to high levels of PFOA via contaminated drinking water in the Mid-Ohio Valley, West Virginia found no association between serum PFOA and incident diabetes [24]. A nested case–control study within the Swedish prospective population-based Västerbotten Intervention Programme cohort (124 case–control pairs; mean age 46 years; 42% female sex; PFAS concentrations from plasma collected in 1990–2003) found non-significant inverse associations [23]. It is not clear why our findings are similar to those of the NHS-II but different from these other studies. Similar population characteristics such as age (midlife) and sex (women), and exposure assessment timing (blood collected around the peak exposure before the population concentrations of PFOS and PFOA started to drop) may explain those discrepancies. If true, this may imply that midlife women may be more susceptible to PFAS-related diabetes risk. Although evidence of sex-dependent associations between PFAS and diabetes in human populations is lacking, a recent study in mice found that female mice had greater hepatic responses to PFOA exposure than male mice [38]. Potential effect modification by age and sex in the association between PFAS and incident diabetes needs to be assessed.
Underlying biological mechanisms of PFAS-induced diabetes risk are not fully understood. PFAS are structurally analogous to fatty acids, well-known endogenous ligands of PPARα and PPARγ. Activation of PPARs by PFOA, PFOS and other substances has been observed in rodents as well as in humans [9]. Human-relevant doses of PFAS have been associated with promotion of adipocyte proliferation and differentiation, which play a pivotal role in disturbed lipid and glucose homeostasis and inflammatory responses [10, 11]. However, activation of PPARα by PFAS, demonstrated in rodent studies to be the primary underlying mechanism, has been questioned in humans because the expression PPARα is low in human liver [39]. Additionally, the fact that exposure to PFOA or PFOS in PPARα-null mice increased hepatic lipid accumulation suggests the involvement of PPARα-independent pathways [39]. Other nuclear receptors such as constitutive androstane receptor and pregnane X receptor, known to alter glucose and lipid metabolisms [40], can be activated by PFAS [39]. Inhibition of the phosphorylation and activation of Akt upon PFOS exposure is another potential mechanism of PFOS-induced insulin resistance [41]. PFOS may also affect glucose homeostasis by interfering with the glucose-lowering effects of metformin and energy restriction [42].
The present study has numerous strengths. First, it evaluated incident diabetes and confounders prospectively for up to 18 years. The prospective design prevented potential reverse causality that could have happened in cross-sectional or prevalent case–control study designs because blood concentrations of PFAS can be influenced by reduced kidney function, which is common in diabetic individuals [19, 20]. Second, our study population included racially/ethnically diverse women, thus increasing the generalisability of our findings. Third, to the best of our knowledge, this is the first study to evaluate the joint effect of PFAS mixtures on diabetes risk. Using the quantile-based g-computation approach [35], we found that the strength of the association for exposure to a combination of PFAS was greater than those for individual compounds.
Several limitations should also be considered. First, we cannot exclude the possibility that the observed associations are due to residual confounding. One potential source of confounding relates to bile acids when exposure to PFAS is assessed as serum concentrations [43]. PFAS are excreted in bile but most PFAS excreted into bile are reabsorbed in the intestine, in turn influencing serum PFAS concentrations. Bile acids play a role in glucose metabolism and insulin signalling through receptor-dependent and independent mechanisms [44]. Therefore, variability in the intestinal reabsorption of both PFAS and bile acids may confound the association between serum concentrations of PFAS and bile acid-dependent health endpoints including diabetes. Second, although we claim that our racial/ethnic diverse female population is a strength of our study, our findings may not be generalisable to male sex, other ages and other ethnicities. Third, while long-chain PFAS have long elimination half-lives, single measurements assessed at baseline may have introduced exposure misclassification in later follow-up visits. However, the potential impact of such bias may be minimal given our observations of high intraclass correlations of repeated measured PFAS serum concentrations over 11 years [30].
In conclusion, this prospective cohort study supports the hypothesis that exposure to PFAS, individually and as mixtures, may increase the risk of incident diabetes in midlife women. Assuming that these results are applicable to men as well as to all ages, locations and races/ethnicities, if serum concentrations of total PFAS were reduced to the fifth percentile of the SWAN participants (15.3 ng/ml), the population attributable fraction for incident diabetes is approximately 25% for total PFAS (see ESM Methods for further details of the population attributable fraction calculation). Given that 1.5 million Americans are newly diagnosed with diabetes each year in the USA [45], approximately 370,000 new cases of diabetes annually in the USA are attributable to PFAS exposure. Our findings suggest that PFAS may be an important risk factor for diabetes that has a substantial public health impact. Despite the population-wide reduction in serum concentrations of PFOS and PFOA [46], almost all people are still exposed to these known ‘forever chemicals’ as well as innumerable alternatives and substitutes (e.g. short-chain PFAS) through drinking water, foods, air and consumer products including cosmetics [47]. Some residents living near industrial sites, military bases and waste-water treatment plants are exposed to substantially higher levels of PFAS [48] and may be at higher risk of developing diabetes. Reduced exposure to these ‘forever and everywhere chemicals’ even before entering midlife may be a key preventative approach to lowering the risk of diabetes. Policy changes around drinking water and consumer products could prevent population-wide exposure. Recent literature suggests that regulations that focus on a few specific PFAS may be ineffective and therefore persistent PFAS may need to be regulated as a class [49]. It is also important for clinicians to be aware of PFAS as unrecognised risk factors for diabetes and to be prepared to counsel patients in terms of sources of exposure and potential health effects [50].
Abbreviations
- CDC:
-
Centers for Disease Control and Prevention
- EtFOSAA:
-
2-(N-ethyl-perfluorooctane sulfonamido) acetic acid
- IPW:
-
Inverse probability weighting
- LOD:
-
Limit of detection
- MeFOSAA:
-
2-(N-methyl-perfluorooctane sulfonamido) acetic acid
- MPS:
-
Multi-Pollutant Study
- NHS-II:
-
Nurses’ Health Study-II
- n-PFOA:
-
Linear PFOA
- n-PFOS:
-
Linear PFOS
- PFAS:
-
Per- and polyfluoroalkyl substances
- PFDA:
-
Perfluorodecanoic acid
- PFDoDA:
-
Perfluorododecanoic acid
- PFHxS:
-
Perfluorohexane sulfonic acid
- PFNA:
-
Perfluorononanoic acid
- PFOA:
-
Perfluorooctanoic acid
- PFOS:
-
Perfluorooctane sulfonic acid
- PFUnDA:
-
Perfluoroundecanoic acid
- PPAR:
-
Peroxisome proliferator-activated receptor
- Sb-PFOA:
-
Sum of branched PFOA isomers
- Sm-PFOS:
-
Sum of perfluoromethylheptane sulfonic acid isomers
- SWAN:
-
Study of Women’s Health Across the Nation
References
Agency for Toxic Substances and Disease Registry (ATSDR) (2021) Toxicological profile for Perfluoroalkyls. U.S. Department of Health and Human Services, Public Health Service. Agency for Toxic Substances and Disease Registry, Atlanta, GA
Interstate Technology and Regulatory Council (ITRC) (2020) History and Use of Per-and Polyfluoroalkyl Substances (PFAS), ITRC, Washington, DC
Andrews DQ, Naidenko OV (2020) Population-wide exposure to per- and Polyfluoroalkyl substances from drinking water in the United States. Environ Sci Technol Lett 7(12):931–936. https://doi.org/10.1021/acs.estlett.0c00713
Centers for Disease Control and Prevention (CDC) (2019) Fourth Report on Human Exposure to Environmental Chemicals, Updated Tables. Centers for Disease Control and Prevention, National Center for Environmental Health. Atlanta, GA
Centers for Disease Control and Prevention (CDC) (2009) Fourth National Report on Human Exposure to Environmental Chemicals. Centers for Disease Control and Prevention, National Center for Environmental Health. Atlanta, GA
Qi W, Clark JM, Timme-Laragy AR, Park Y (2020) Per- and Polyfluoroalkyl substances and obesity, type 2 diabetes and non-alcoholic fatty liver disease: a review of epidemiologic findings. Toxicol Environ Chem 102(1–4):1–36. https://doi.org/10.1080/02772248.2020.1763997
Buck RC, Franklin J, Berger U et al (2011) Perfluoroalkyl and polyfluoroalkyl substances in the environment: terminology, classification, and origins. Integr Env Assess Manag 7(4):513–541. https://doi.org/10.1002/ieam.258
Grygiel-Górniak B (2014) Peroxisome proliferator-activated receptors and their ligands: nutritional and clinical implications--a review. Nutr J 13:17. https://doi.org/10.1186/1475-2891-13-17
Jiang Q, Gao H, Zhang L (2015) Chapter 7. Metabolic effects PFAS. In: DeWitt JC (ed) Toxicological effects of Perfl uoroalkyl and Polyfluoroalkyl substances. Springer International Publishing, Switzerland
Watkins AM, Wood CR, Lin MT, Abbott BD (2015) The effects of perfluorinated chemicals on adipocyte differentiation in vitro. Mol Cell Endocrinol 400:90–101. https://doi.org/10.1016/j.mce.2014.10.020
Liu S, Yang R, Yin N, Wang Y-L, Faiola F (2019) Environmental and human relevant PFOS and PFOA doses alter human mesenchymal stem cell self-renewal, adipogenesis and osteogenesis. Ecotoxicol Environ Saf 169:564–572. https://doi.org/10.1016/j.ecoenv.2018.11.064
Lin CY, Chen PC, Lin YC, Lin LY (2009) Association among serum perfluoroalkyl chemicals, glucose homeostasis, and metabolic syndrome in adolescents and adults. Diabetes Care 32(4):702–707. https://doi.org/10.2337/dc08-1816
MacNeil J, Steenland NK, Shankar A, Ducatman A (2009) A cross-sectional analysis of type II diabetes in a community with exposure to perfluorooctanoic acid (PFOA). Environ Res 109(8):997–1003. https://doi.org/10.1016/j.envres.2009.08.002
Lind L, Zethelius B, Salihovic S, van Bavel B, Lind PM (2014) Circulating levels of perfluoroalkyl substances and prevalent diabetes in the elderly. Diabetologia 57(3):473–479. https://doi.org/10.1007/s00125-013-3126-3
Su T-C, Kuo C-C, Hwang J-J, Lien G-W, Chen M-F, Chen P-C (2016) Serum perfluorinated chemicals, glucose homeostasis and the risk of diabetes in working-aged Taiwanese adults. Environ Int 88:15–22. https://doi.org/10.1016/j.envint.2015.11.016
Zeeshan M, Zhang Y-T, Yu S et al (2021) Exposure to isomers of per- and polyfluoroalkyl substances increases the risk of diabetes and impairs glucose-homeostasis in Chinese adults: isomers of C8 health project. Chemosphere 278:130486. https://doi.org/10.1016/j.chemosphere.2021.130486
Duan Y, Sun H, Yao Y et al (2021) Serum concentrations of per−/polyfluoroalkyl substances and risk of type 2 diabetes: a case-control study. Sci Total Environ 787:147476. https://doi.org/10.1016/j.scitotenv.2021.147476
Han X, Meng L, Zhang G et al (2021) Exposure to novel and legacy per- and polyfluoroalkyl substances (PFASs) and associations with type 2 diabetes: a case-control study in East China. Environ Int 156:106637. https://doi.org/10.1016/j.envint.2021.106637
Dhingra R, Winquist A, Darrow LA, Klein M, Steenland K (2017) A study of reverse causation: examining the associations of Perfluorooctanoic acid serum levels with two outcomes. Env Heal Perspect 125(3):416–421. https://doi.org/10.1289/EHP273EHP273
Park SK, Ding N, Han D (2021) Perfluoroalkyl substances and cognitive function in older adults: should we consider non-monotonic dose-responses and chronic kidney disease? Environ Res 192:110346. https://doi.org/10.1016/j.envres.2020.110346
Sun Q, Zong G, Valvi D, Nielsen F, Coull B, Grandjean P (2018) Plasma concentrations of Perfluoroalkyl substances and risk of type 2 diabetes: a prospective investigation among U.S. women. Environ Health Perspect 126(3):037001. https://doi.org/10.1289/EHP2619
Cardenas A, Hivert M-F, Gold DR et al (2019) Associations of Perfluoroalkyl and Polyfluoroalkyl substances with incident diabetes and microvascular disease. Diabetes Care 42(9):1824–1832. https://doi.org/10.2337/dc18-2254
Donat-Vargas C, Bergdahl IA, Tornevi A et al (2019) Perfluoroalkyl substances and risk of type II diabetes: a prospective nested case-control study. Environ Int 123:390–398. https://doi.org/10.1016/j.envint.2018.12.026
Karnes C, Winquist A, Steenland K (2014) Incidence of type II diabetes in a cohort with substantial exposure to perfluorooctanoic acid. Environ Res 128:78–83. https://doi.org/10.1016/j.envres.2013.11.003
Park SK, Zhao Z, Mukherjee B (2017) Construction of environmental risk score beyond standard linear models using machine learning methods: application to metal mixtures, oxidative stress and cardiovascular disease in NHANES. Environ Heal A Glob Access Sci Source 16(1):102. https://doi.org/10.1186/s12940-017-0310-9
Braun JM, Gennings C, Hauser R, Webster TF (2016) What can epidemiological studies tell us about the impact of chemical mixtures on human health? Env Heal Perspect 124(1):A6–A9. https://doi.org/10.1289/ehp.1510569
Sowers MR, Crawford SL, Sternfeld B et al (2000) SWAN: a multicenter, multiethnic, community-based cohort study of women and the menopausal transition. In: Lobo RA, Kelsey J, Marcus R (eds) Menopause: biology and pathology. Academic Press, San Diego, CA, pp 175–188
Kato K, Basden BJ, Needham LL, Calafat AM (2011) Improved selectivity for the analysis of maternal serum and cord serum for polyfluoroalkyl chemicals. J Chromatogr A 1218(15):2133–2137. https://doi.org/10.1016/j.chroma.2010.10.051S0021-9673(10)01427-5
Park SK, Peng Q, Ding N, Mukherjee B, Harlow SD (2019) Determinants of per- and polyfluoroalkyl substances (PFAS) in midlife women: evidence of racial/ethnic and geographic differences in PFAS exposure. Environ Res 175:186–199. https://doi.org/10.1016/j.envres.2019.05.028
Ding N, Harlow SD, Batterman S, Mukherjee B, Park SK (2020) Longitudinal trends in perfluoroalkyl and polyfluoroalkyl substances among multiethnic midlife women from 1999 to 2011: the study of Women’s health across the nation. Environ Int 135:105381. https://doi.org/10.1016/j.envint.2019.105381
EFSA (European Food Safety Authority) (2020) Outcome of a public consultation on the draft risk assessment of perfluoroalkyl substances in food. EFSA Support Publ 2020EN-1931. https://doi.org/10.2903/SP.EFSA.2020.EN-1931
Ding N, Karvonen-Gutierrez CA, Herman WH, Calafat AM, Mukherjee B, Park SK (2021) Perfluoroalkyl and polyfluoroalkyl substances and body size and composition trajectories in midlife women: the study of women’s health across the nation 1999-2018. Int J Obes 45(9):1937–1948. https://doi.org/10.1038/s41366-021-00848-9
Ding N, Harlow SD, Randolph JF et al (2020) Associations of Perfluoroalkyl substances with incident natural menopause: the study of Women’s health across the nation. J Clin Endocrinol Metab 105(9):e3169–e3182. https://doi.org/10.1210/clinem/dgaa303
Zheng Y, Ley SH, Hu FB (2018) Global aetiology and epidemiology of type 2 diabetes mellitus and its complications. Nat Rev Endocrinol 14(2):88–98. https://doi.org/10.1038/nrendo.2017.151
Keil AP, Buckley JP, O’Brien KM, Ferguson KK, Zhao S, White AJ (2020) A quantile-based g-computation approach to addressing the effects of exposure mixtures. Environ Health Perspect 128(4):47004. https://doi.org/10.1289/EHP5838
Abdullah A, Peeters A, de Courten M, Stoelwinder J (2010) The magnitude of association between overweight and obesity and the risk of diabetes: a meta-analysis of prospective cohort studies. Diabetes Res Clin Pract 89(3):309–319. https://doi.org/10.1016/J.DIABRES.2010.04.012
Pan A, Wang Y, Talaei M, Hu FB, Wu T (2015) Relation of active, passive, and quitting smoking with incident diabetes: a meta-analysis and systematic review. Lancet Diabetes Endocrinol 3(12):958. https://doi.org/10.1016/S2213-8587(15)00316-2
Schlezinger JJ, Puckett H, Oliver J, Nielsen G, Heiger-Bernays W, Webster TF (2020) Perfluorooctanoic acid activates multiple nuclear receptor pathways and skews expression of genes regulating cholesterol homeostasis in liver of humanized PPARα mice fed an American diet. Toxicol Appl Pharmacol 405:115204. https://doi.org/10.1016/j.taap.2020.115204
Fragki S, Dirven H, Fletcher T et al (2021) Systemic PFOS and PFOA exposure and disturbed lipid homeostasis in humans: what do we know and what not? Crit Rev Toxicol 51(2):141–164. https://doi.org/10.1080/10408444.2021.1888073
Moreau A, Vilarem MJ, Maurel P, Pascussi JM (2008) Xenoreceptors CAR and PXR activation and consequences on lipid metabolism, glucose homeostasis, and inflammatory response. Mol Pharm 5(1):35–41. https://doi.org/10.1021/mp700103m
Qiu T, Chen M, Sun X et al (2016) Perfluorooctane sulfonate-induced insulin resistance is mediated by protein kinase B pathway. Biochem Biophys Res Commun 477(4):781–785. https://doi.org/10.1016/j.bbrc.2016.06.135
Salter DM, Wei W, Nahar PP, Marques E, Slitt AL (2021) Perfluorooctanesulfonic acid (PFOS) thwarts the beneficial effects of calorie restriction and metformin. Toxicol Sci 182(1):82–95. https://doi.org/10.1093/toxsci/kfab043
Li Y, Barregard L, Xu Y et al (2020) Associations between perfluoroalkyl substances and serum lipids in a Swedish adult population with contaminated drinking water. Environ Health 19(1):33. https://doi.org/10.1186/s12940-020-00588-9
Ahmad TR, Haeusler RA (2019) Bile acids in glucose metabolism and insulin signalling — mechanisms and research needs. Nat Rev Endocrinol 15(12):701–712. https://doi.org/10.1038/s41574-019-0266-7
Centers for Disease Control and Prevention (CDC) (2020) National Diabetes Statistics Report, 2020. Centers for Disease Control and Prevention, National Center for Environmental Health. Atlanta, GA
Kato K, Wong LY, Jia LT, Kuklenyik Z, Calafat AM (2011) Trends in exposure to polyfluoroalkyl chemicals in the U.S. population: 1999-2008. Env Sci Technol 45(19):8037–8045. https://doi.org/10.1021/es1043613
Whitehead HD, Venier M, Wu Y et al (2021) Fluorinated compounds in north American cosmetics. Environ Sci Technol Lett 8(7):538–544. https://doi.org/10.1021/acs.estlett.1c00240
Hu XC, Andrews DQ, Lindstrom AB et al (2016) Detection of poly- and Perfluoroalkyl substances (PFASs) in U.S. drinking water linked to industrial sites, military fire training areas, and wastewater treatment plants. Env Sci Technol Lett 3(10):344–350. https://doi.org/10.1021/acs.estlett.6b00260
Cousins IT, DeWitt JC, Glüge J et al (2020) The high persistence of PFAS is sufficient for their management as a chemical class. Environ Sci Process Impacts 22(12):2307–2312. https://doi.org/10.1039/d0em00355g
Agency for Toxic Substances and Disease Registry (ATSDR) (2019) PFAS: An overview of the science and guidance for clinicians on per- and polyfluoroalkyl substances (PFAS). U.S. Department of Health and Human Services, Public Health Service. Agency for Toxic Substances and Disease Registry. Atlanta, GA
Acknowledgements
We thank all the women who participated in SWAN. We also thank the late Xiaoyun Ye at CDC for her support in PFAS assessment. We thank the study staff and principal investigators at each of the following sites.
Clinical centres: University of Michigan, Ann Arbor, MI (C. Karvonen-Gutierrez, S. Harlow, M. Sowers); Massachusetts General Hospital, Boston, MA (J. Finkelstein, R. Neer); Rush University, Rush University Medical Center, Chicago, IL (H. Kravitz, L. Powell); University of California, Davis/Kaiser (E. Gold); University of California, Los Angeles (G. Greendale); Albert Einstein College of Medicine, Bronx, NY (C. Derby, R. Wildman, N. Santoro); University of Medicine and Dentistry, New Jersey Medical School, Newark, NJ (G. Weiss); and the University of Pittsburgh, Pittsburgh, PA (K. Matthews).
National Institutes of Health (NIH) Program Office: National Institute on Aging, Bethesda, MD (C. Dutta, W. Rossi, S. Sherman, M. Ory); National Institute of Nursing Research, Bethesda, MD (Program Officers).
Central Laboratory: Central Ligand Assay Satellite Services, University of Michigan, Ann Arbor, MI (D. McConnell).
CDC Laboratory: Division of Laboratory Sciences, National Center for Environmental Health, Centers for Disease Control and Prevention, Atlanta, GA.
SWAN Repository: University of Michigan, Ann Arbor, MI (S. Harlow, D. McConnell, M. Sowers).
Coordinating Center: University of Pittsburgh, Pittsburgh, PA (M. Mori Brooks, K. Sutton-Tyrrell); New England Research Institutes, Watertown, MA (S. McKinlay).
Steering Committee: S. Johnson, C. Gallagher.
Data availability
SWAN provides access to public-use datasets that include data from SWAN screening, the baseline visit and follow-up visits (https://agingresearchbiobank.nia.nih.gov/). To preserve participant confidentiality, some, but not all, of the data used for this manuscript are contained in the public-use datasets. A link to the public-use datasets is also located on the SWAN website: http://www.swanstudy.org/swan-research/data-access/. Investigators who require assistance accessing the public use dataset may contact the SWAN Coordinating Center at the following e-mail address: swanaccess@edc.pitt.edu.
Funding
SWAN has grant support from the NIH, US Department of Health and Human Services (DHHS), through the National Institute on Aging (NIA), the National Institute of Nursing Research (NINR) and the NIH Office of Research on Women’s Health (ORWH) (grants U01NR004061, U01AG012505, U01AG012535, U01AG012531, U01AG012539, U01AG012546, U01AG012553, U01AG012554, U01AG012495 and U19AG063720). The study was also supported by the SWAN Repository (U01AG017719). This publication was supported in part by the National Center for Research Resources and the National Center for Advancing Translational Sciences, NIH, through UCSF-CTSI grant no. UL1 RR024131. This study was also supported by grants from the National Institute of Environmental Health Sciences (NIEHS) R01-ES026578, R01-ES026964 and P30-ES017885, and by the CDC/National Institute for Occupational Safety and Health (NIOSH) grant T42-OH008455. The content of this article is solely the responsibility of the authors and does not necessarily represent the official views of the NIA, NINR, ORWH or the NIH. The findings and conclusions of this report are those of the authors and do not necessarily represent the official position of the CDC. Use of trade names is for identification only and does not imply endorsement by the CDC, the Public Health Service or the DHHS.
Authors’ relationships and activities
The authors declare that there are no relationships or activities that might bias, or be perceived to bias, their work.
Contribution statement
SKP was involved in the design of the analysis plan and wrote the manuscript. XW and ND conducted data analyses and critically revised the manuscript. CAKG, WHH, BM, AMC and SDH were involved in the design of the analysis plan, contributed to interpretation of the data, and critically revised the manuscript. All authors read and approved the final version of the paper. SKP is the guarantor of this work and had full access to all the data in the study and takes responsibility for the contents of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
ESM
(PDF 314 kb)
Rights and permissions
About this article
Cite this article
Park, S.K., Wang, X., Ding, N. et al. Per- and polyfluoroalkyl substances and incident diabetes in midlife women: the Study of Women’s Health Across the Nation (SWAN). Diabetologia 65, 1157–1168 (2022). https://doi.org/10.1007/s00125-022-05695-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00125-022-05695-5