Abstract
Aims/hypothesis
It has been proposed that muscle fibre type composition and perfusion are key determinants of insulin-stimulated muscle glucose uptake, and alterations in muscle fibre type composition and perfusion contribute to muscle, and consequently whole-body, insulin resistance in people with obesity. The goal of the study was to evaluate the relationships among muscle fibre type composition, perfusion and insulin-stimulated glucose uptake rates in healthy, lean people and people with obesity.
Methods
We measured insulin-stimulated whole-body glucose disposal and glucose uptake and perfusion rates in five major muscle groups (erector spinae, obliques, rectus abdominis, hamstrings, quadriceps) in 15 healthy lean people and 37 people with obesity by using the hyperinsulinaemic–euglycaemic clamp procedure in conjunction with [2H]glucose tracer infusion (to assess whole-body glucose disposal) and positron emission tomography after injections of [15O]H2O (to assess muscle perfusion) and [18F]fluorodeoxyglucose (to assess muscle glucose uptake). A biopsy from the vastus lateralis was obtained to assess fibre type composition.
Results
We found: (1) a twofold difference in glucose uptake rates among muscles in both the lean and obese groups (rectus abdominis: 67 [51, 78] and 32 [21, 55] μmol kg−1 min−1 in the lean and obese groups, respectively; erector spinae: 134 [103, 160] and 66 [24, 129] μmol kg−1 min−1, respectively; median [IQR]) that was unrelated to perfusion or fibre type composition (assessed in the vastus only); (2) the impairment in insulin action in the obese compared with the lean group was not different among muscle groups; and (3) insulin-stimulated whole-body glucose disposal expressed per kg fat-free mass was linearly related with muscle glucose uptake rate (r2 = 0.65, p < 0.05).
Conclusions/interpretation
Obesity-associated insulin resistance is generalised across all major muscles, and is not caused by alterations in muscle fibre type composition or perfusion. In addition, insulin-stimulated whole-body glucose disposal relative to fat-free mass provides a reliable index of muscle glucose uptake rate.
Graphical abstract

Similar content being viewed by others

Introduction
Insulin-stimulated glucose uptake in skeletal muscles is important for maintaining plasma glucose homeostasis. Impaired insulin-stimulated muscle glucose uptake is common in people with obesity and is a major risk factor for type 2 diabetes [1, 2]. Insulin promotes muscle glucose uptake through several mechanisms, including an insulin-mediated increase in muscle perfusion and concomitant increase in both insulin and glucose delivery to muscles, increased GLUT4 translocation to the plasma membrane to facilitate glucose transport into myocytes and intracellular glucose phosphorylation [3]. In rodent models, insulin-stimulated glucose uptake rate, assessed both in vivo and ex vivo, varies several-fold among different muscles and is generally greater in muscles that contain primarily type I fibres than in those that contain primarily fast glycolytic type II fibres, presumably because of greater capillary density (and subsequently perfusion) and GLUT4 content in type I fibre-rich muscles [4,5,6]. In addition, the susceptibility to lipid- and high-fat diet-induced insulin resistance differs among different myofibre types and muscles in rodent models. High-fat feeding impairs insulin-stimulated glucose uptake in isolated type IIx, but not type I and type IIa, fibres [7], and lipid infusion and high-fat feeding impair insulin-stimulated glucose uptake in some but not all muscles [8, 9]. However, it is not known whether insulin-stimulated glucose uptake rates differ among different muscles in people and whether obesity causes insulin resistance in specific muscle groups only, because muscle insulin sensitivity is typically assessed as the glucose infusion rate needed to maintain euglycaemia during a hyperinsulinaemic–euglycaemic clamp procedure or as insulin-stimulated whole-body or limb glucose disposal rate [2, 10,11,12,13].
The primary goal of the present study was to evaluate: (1) glucose uptake rates in different skeletal muscles in healthy lean people (lean group) and people with obesity (obese group); and (2) the relationships among muscle perfusion, fibre type composition and glucose uptake. A secondary goal was to evaluate the common assumption that insulin-stimulated whole-body glucose disposal is a reliable surrogate measure of muscle glucose uptake [2, 10, 12, 13]. To this end, we measured insulin-stimulated whole-body glucose disposal and skeletal muscle perfusion and glucose uptake rates by using a hyperinsulinaemic–euglycaemic pancreatic clamp procedure in conjunction with [6,6-2H2]glucose tracer infusion and positron emission tomography (PET) after bolus injections of [15O]H2O and [18F]fluorodeoxyglucose ([18F]FDG). Specific muscle groups in the torso (erector spinae, obliques, rectus abdominis) and thigh (hamstrings, quadriceps) were studied because they differ in their fibre type composition; muscles along the spine have more type I fibres than abdominal muscles, muscles in the torso have more type I fibres than leg muscles and the hamstrings have more type I fibres than the quadriceps [14, 15]. We hypothesised that: (1) insulin-stimulated muscle glucose uptake rate varies among different muscle groups as a function of muscle perfusion and fibre type composition and is therefore greater in the erector spinae than abdominal muscles, greater in muscles in the torso than leg muscles and greater in the hamstrings than the quadriceps; and (2) obesity-associated muscle insulin resistance is less pronounced in muscles of the torso than of the leg. Moreover, we hypothesised that insulin-stimulated glucose uptake rate in the vastus lateralis correlates with vastus lateralis fibre type composition.
Methods
Study participants
The data reported here were obtained from 15 healthy lean people (five men, ten women; 39 ± 3 years, mean ± SEM) and 37 people with obesity (nine men, 28 women; 44 ± 1 years) who participated in two different, currently ongoing, larger studies that used the same experimental protocol (ClinicalTrials.gov no. NCT02994459 and no. NCT03408613), which was approved by the Human Research Protection Office at Washington University School of Medicine in St Louis, MO, USA. All participants completed a dual-energy x-ray absorptiometry (DXA, Lunar iDXA, GE Healthcare Lunar, Madison, WI, USA) scan to determine body composition and a comprehensive medical examination, including a history and physical examination, a resting electrocardiogram, standard blood tests and an oral glucose tolerance test. All participants were sedentary (<1.5 h of exercise/week), and none had evidence of chronic illness or significant organ dysfunction (including type 2 diabetes), took medications that interfere with insulin action or glucose metabolism, or consumed tobacco products and/or excessive amounts of alcohol. Written, informed consent was obtained from all participants before their participation.
Hyperinsulinaemic–euglycaemic pancreatic clamp procedure
Participants were admitted to the Clinical Translational Research Unit the night before the study. At 20:00 hours, they consumed a standardised meal and then fasted, except for water, until the completion of the study the next day. At ~06:00 hours, a catheter was inserted into an antecubital vein to infuse the metabolic tracers, hormones and dextrose; another catheter was inserted into a radial artery for blood sampling. Participants were then transferred to the Center for Clinical Imaging Research, where constant infusions of octreotide (45 ng [kg fat-free mass (FFM)]−1 min−1), glucagon (1.5 ng [kg FFM]−1 min−1) and growth hormone (6 ng [kg FFM]−1 min−1) were started and maintained for 390 min. Insulin was infused at 10 mU (m2 body surface area)−1 min−1 for the first 120 min then at 50 mU (m2 body surface area)−1 min−1, both initiated with a two-step priming dose; [6,6-2H2]glucose was infused at 0.22 μmol (kg body weight)−1 min−1 for the first 120 min, and then at 0.11 μmol (kg body weight)−1 min−1 [10, 16]. Dextrose, enriched with [6,6-2H2]glucose (2.5%), was infused at a variable rate to maintain plasma glucose concentration (monitored every 10 min) at ~6.1 mmol/l during the hyperinsulinaemic clamp procedure. We chose a pancreatic clamp procedure to minimise differences in plasma insulin concentration during the clamp procedure in healthy lean people and people with obesity [10, 11]. Approximately 270 min after starting the hyperinsulinaemic clamp procedure, participants were transferred to the PET/computed tomography (CT) scanner (Siemens Biograph True Point/True View, Siemens Medical Solutions, USA), where ~1.2 GBq [15O]H2O was administered as a bolus and 2 min of dynamic PET scanning of the torso was performed. Participants were then quickly repositioned and a second dose of [15O]H2O was administered followed by 2 min of PET scanning of the thigh. At ~300 min, ~185 MBq [18F]FDG was administered intravenously and 40 min of dynamic PET scanning of the torso, followed by 30 min of dynamic PET scanning of the thigh, was performed. Low-dose CT scans (120 kVp, 50 mAs effective) were performed for attenuation correction and to delineate the muscle regions of interest. Blood samples to determine plasma insulin concentration (Elecsys, Roche Diagnostics, USA) were collected immediately before the start of the hormone infusions and then every 10 min from 300 to 390 min. A biopsy from the vastus lateralis was obtained during local anaesthesia from 13 of the lean participants and 28 of the participants with obesity for histology.
Muscle histology
Muscle biopsies were embedded in tragacanth gum, flash frozen in liquid nitrogen-cooled isopentane and then stored at −80°C. Frozen samples were cut in the axial plane in 10 μm sections, which were immunostained against type I, IIa and IIx fibres (Developmental Studies Hybridoma Bank: BA-F8, SC-71 and 6H1, respectively; University of Iowa, USA). Additional sections were stained with fluorescin-conjugated lectin (Vector Laboratories FL-1061; Maravai LifeSciences, USA) to visualise capillaries and counterstained with laminin (ab11575; Abcam, USA) and 4′,6-diamidino-2-phenylindole to visualise fibre boundaries and nuclei, respectively. Image analysis was performed with the public domain software ImageJ [17].
Calculations
Muscle perfusion (i.e. capillary blood flow rate in ml [kg muscle]–1 min–1) was determined by using a one-compartment model to fit the arterial blood (corrected for spillover and partial volume) and muscle specific activity–time courses after [15O]H2O injection [18]. Glucose delivery to muscles was calculated as the product of muscle capillary blood flow rate and circulating glucose concentration. The fractional glucose uptake rate for each muscle group was calculated by using Patlak graphical analysis of the muscle and arterial blood specific activity–time curves [19, 20]. Absolute rates of glucose uptake (μmol [kg muscle]–1 min–1) were derived by dividing the product of fractional glucose uptake rate and plasma glucose concentration by 1.2 (lumped constant) [19]. Total whole-body muscle glucose disposal rate was estimated as the product of the mean glucose uptake rates in the erector spinae, obliques, rectus abdominis, hamstrings and quadriceps, and muscle mass, which was assumed to be 53% of lean soft tissue mass [21]. We used both the arithmetic mean and the weighted mean for this estimation and found it made no difference; the arithmetic mean and weighted mean glucose uptake rates (mean ± SEM) in the five muscles were 100 ± 7 and 103 ± 9 μmol (kg muscle)−1 min−1 in lean people and 63 ± 7 and 67 ± 8 μmol (kg muscle)−1 min−1 in people with obesity, respectively. We therefore only present the arithmetic mean values. Unlabelled glucose rate of appearance in plasma (endogenous glucose production and dextrose solution) was calculated by dividing the [2H2]glucose infusion rate (sum of [2H2]glucose infusion and [2H2]glucose in the labelled dextrose solution) by the mean plasma glucose tracer-to-tracee ratio [16]. Whole-body glucose disposal rate was calculated as the sum of unlabelled glucose appearance rate and glucose tracer infusion rate.
Statistical analysis
Statistical analyses were performed by using Prism 8 (GraphPad Software, San Diego, CA, USA). All datasets were tested for normality by using the Shapiro–Wilk test. Skewed datasets were log transformed before analysis, and transformation resulted in normally distributed datasets. Accordingly, differences in muscle perfusion and muscle glucose uptake rates between lean and obese participants were evaluated by using ANOVA with group and muscle as factors. This analysis identified an effect of obesity, but no obesity × muscle interaction. Tukey’s post hoc procedure, which adjusts for multiple comparisons, was used to locate differences in both perfusion and glucose uptake rates among muscle groups. The relationships among: (1) glucose uptake rates in different muscle groups; (2) muscle perfusion and glucose uptake rate; (3) fibre type composition and glucose uptake rate in the vastus lateralis; and (4) muscle glucose uptake rate and whole-body glucose disposal rate were evaluated by using regression analysis to identify the best fit and the strength of the association. We also explored whether age, sex and BMI affect the associations and found they did not improve the model fits. We therefore present the r2 values from simple regression analysis. Bonferroni’s correction was used to account for multiple comparisons. Values are reported as mean ± SEM or median and interquartile range. A p value <0.05 was considered statistically significant.
Results
Participant characteristics
Participants’ body composition and basic metabolic characteristics are shown in Table 1. Body fat mass was more than double in the obese than in the lean group and FFM was ~25% greater in the obese than in the lean group. Fasting plasma glucose concentration was not different in the lean and obese groups, but glucose tolerance was markedly impaired in the obese compared with the lean group. Plasma insulin, triacylglycerol and LDL-cholesterol concentrations were higher and HDL-cholesterol concentration was lower in the obese than in the lean group.
Plasma glucose and insulin concentrations during the hyperinsulinaemic–euglycaemic pancreatic clamp procedure
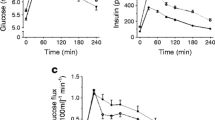
Steady-state plasma glucose and insulin concentrations during the clamp procedure were not different in the lean and obese groups (glucose: 6.2 ± 0.1 and 6.1 ± 0.1 mmol/l, respectively; insulin: 638 ± 31 and 697 ± 15 pmol/l, respectively).
Muscle perfusion (capillary blood flow rate)
Muscle perfusion rates were up to five times different among different muscle groups in both the lean and the obese groups (Fig. 1). Muscle perfusion rates were several-fold higher in the erector spinae, hamstrings and quadriceps than the rectus abdominis and obliques (Fig. 1). Perfusion rates were not statistically significantly different in the lean and obese groups (Fig. 1).
Insulin-stimulated muscle perfusion (a, b) and glucose uptake (c, d) rates in major muscle groups of the torso and thigh in healthy lean people (a, c; n=15, white bars and symbols) and people with obesity (b, d; n=37, orange bars and symbols). Values are median and interquartile range. *p<0.05 vs erector spinae, hamstrings and quadriceps; †p<0.05 vs erector spinae; ‡p<0.05 vs erector spinae and hamstrings; §p<0.05, significantly different from corresponding values in lean people
Muscle glucose uptake
On average, only a small fraction (<20%; overall median and interquartile range: 6.9 [3.0, 12.2]) of glucose delivered to muscles was taken up by muscles in both the lean and obese groups. Glucose uptake rate in the erector spinae was about twice as great as in the rectus abdominis and approximately 30% greater than in the obliques and quadriceps (Fig. 1). There was considerable variability in glucose uptake rates in the obese group, but, on average, glucose uptake rates were ~50% less in the obese than in the lean group (Fig. 1), without a difference among muscle groups (erector spinae: 49% [18%, 97%], obliques: 38% [30%, 84%], rectus abdominis: 49% [32%, 83%], hamstrings: 53% [28%, 96%], quadriceps: 49% [27%, 73%]; values in obese participants relative to mean lean value). The results were the same when muscle glucose uptake rates were expressed in relation to plasma insulin concentration during the clamp procedure (data not shown). There were linear relationships among glucose uptake rates in different muscle groups without a difference between the lean and obese groups (Fig. 2 and Electronic supplementary material [ESM] Fig. 1). There was no association between muscle perfusion and glucose uptake rates in any single muscle group or in all muscles combined (ESM Fig. 2).
Vastus lateralis morphology and relationship between fibre type composition and glucose uptake rate in the vastus lateralis
There was considerable interindividual variation in vastus lateralis fibre type composition in both the lean and obese groups. On average, type I fibres accounted for ~40% and type II fibres accounted for ~60% of all fibres in both the lean and obese groups. The contribution of type IIx fibres to total fibre number was double in the obese compared with the lean group, and type IIx fibres replaced both type I and type IIa fibres proportionally (Table 2). There was also considerable interindividual variation in the number of capillaries in the vastus lateralis in both the lean and obese groups, and, on average, capillary density (both per cross-sectional area and per fibre) was not different in the lean than in the obese group (Table 2). There was no association between the proportion of type I, IIa or IIx fibres and glucose uptake rate in the vastus lateralis in either the lean or the obese groups or the entire study cohort (ESM Fig. 3).
Whole-body glucose kinetics and relationship between whole-body glucose kinetics and muscle glucose uptake rates
The glucose infusion rate needed to maintain euglycaemia and insulin-stimulated whole-body glucose disposal rate were significantly lower in the obese than in the lean group, and the relative obesity-associated reduction in insulin sensitivity varied from ~30% to ~70% according to how the data were expressed (i.e. as total flux, in μmol/min, or adjusted for differences in body size or composition) (Table 3). There was a linear relationship (r2 = 0.72, p < 0.05) between total muscle glucose uptake (expressed in μmol/min) and total whole-body glucose disposal rate (expressed in μmol/min), without a difference between the lean and obese groups. However, total muscle glucose uptake was lower than whole-body glucose disposal, and accounted for only ~60% of whole-body glucose disposal in both the lean (57% ± 5%) and the obese (56% ± 4%) groups (Fig. 3). There was also a linear relationship (r2 = 0.83, p < 0.05) between muscle glucose uptake rate (expressed in μmol [kg muscle]–1 min–1) and whole-body glucose disposal rate in relation to total body weight (expressed in μmol [kg body weight]–1 min–1) in the obese but not the lean group. However, muscle glucose uptake rate was greater than whole-body glucose disposal rate per kg body weight (Fig. 3). There was a linear relationship (r2 = 0.65, p < 0.05) between muscle glucose uptake rate (expressed in μmol [kg muscle]–1 min–1) and whole-body glucose disposal rate in relation to FFM (expressed in μmol [kg FFM]–1 min–1). Moreover, the values for whole-body glucose disposal in relation to FFM nearly matched the values for muscle glucose uptake rate in both the lean and obese groups, without a difference between the groups (Fig. 3). The relationships between the glucose infusion rate needed to maintain euglycaemia, expressed as total flux (in μmol/min), or adjusted for differences in body size or composition (expressed in μmol [kg body weight]–1 min–1 or μmol [kg FFM]–1 min–1), and the mean muscle glucose uptake rate (expressed in μmol [kg muscle]–1 min–1) mirrored the relationships between whole-body glucose disposal rate and muscle glucose uptake rate (ESM Fig. 4).
Relationships between insulin-stimulated total muscle glucose uptake (product of muscle mass and mean glucose uptake rate in the erector spinae, obliques, rectus abdominis, hamstrings and quadriceps) and whole-body glucose disposal rate (a), and between the composite mean insulin-stimulated glucose uptake rate in the five muscle groups and whole-body glucose disposal rate in relation to body weight (b), and FFM (c) in healthy lean people (open circles; n=15) and people with obesity (orange filled circles; n=37). Dashed lines represent the lines of identity
Discussion
Insulin-stimulated whole-body glucose disposal is commonly used as an assessment of insulin-stimulated muscle glucose uptake [2, 10, 12, 13]. However, it does not provide a direct measure of insulin sensitivity in skeletal muscles and prevents an assessment of whether some muscles are more important than others in causing whole-body insulin resistance in people with obesity; it also prevents an assessment of how muscle perfusion and fibre type composition affect muscle glucose uptake. We used a hyperinsulinaemic–euglycaemic clamp procedure in conjunction with stable isotope- and radio-labelled tracer infusions and PET technology to evaluate insulin-stimulated whole-body glucose disposal and muscle perfusion, glucose delivery and glucose uptake rates in five major muscle groups in the torso and thigh. We found considerable heterogeneity in insulin-stimulated glucose uptake rates among individual muscle groups that was not due to differences in muscle perfusion (insulin and glucose delivery) or fibre type composition (assessed in the vastus only). We also found that the impairment in skeletal muscle insulin-stimulated glucose uptake in people with obesity is generalised across all major muscles, without specific defects in any particular muscle group, and that insulin-stimulated whole-body glucose disposal rate expressed in relation to FFM correlated well with insulin-stimulated glucose uptake rates in the five muscle groups.
It has been proposed that whole-body insulin resistance in people with obesity is due to alterations in muscle fibre type based on the following observations: (1) fibre type composition is an important determinant of insulin-stimulated muscle glucose uptake in rodent muscles, and type IIx fibres isolated from rodent muscles are more insulin resistant than isolated type I fibres [4,5,6,7]; (2) people with obesity have more type IIx fibres than lean people [22]; and (3) studies conducted in people with a wide range of adiposity have shown that whole-body insulin-stimulated glucose disposal is directly correlated with the proportion of type I fibres and inversely correlated with the proportion of type IIx fibres in the vastus lateralis [23, 24]. However, the results from these studies in people do not prove that the effect of fibre type on muscle insulin sensitivity observed in rodents translates to physiologically meaningful effects of fibre type composition on muscle insulin sensitivity in people. It has even been proposed that insulin resistance and hyperinsulinaemia cause the increase in type IIx fibres in people with insulin resistance [25, 26]. We are not aware of any studies that directly evaluated the effect of fibre type on insulin-stimulated muscle glucose uptake in specific muscles in people. In the present study, we measured glucose uptake in muscle groups that are known to differ in fibre type composition; muscles along the spine (erector spinae) have more type I fibres than abdominal muscles (rectus abdominis, obliques), muscles in the torso have more type I fibres than thigh muscles (hamstrings, quadriceps) and the hamstrings have more type I fibres than the quadriceps [14, 15]. We also obtained muscle biopsies from the vastus lateralis so that we could directly evaluate the relationship between measured fibre type composition and insulin-stimulated muscle glucose uptake rate in that muscle. Our data do not support an influence of muscle fibre type composition on muscle insulin sensitivity. Although glucose uptake in the erector spinae was greater than in abdominal muscles, glucose uptake in abdominal muscles was not different from or was even lower than glucose uptake in thigh muscles, and glucose uptake in the hamstrings was not different from glucose uptake in the quadriceps. Moreover, we did not find an association between measured fibre type composition and insulin-stimulated glucose uptake in the vastus lateralis. These data demonstrate that muscle fibre type composition is not an important determinant of insulin-stimulated muscle glucose disposal, and that glucose uptake is increased in postural back muscles and weight-bearing leg muscles, possibly because insulin-stimulated muscle glucose uptake is more closely related to habitual muscle use [3, 27, 28] than to fibre type. These findings are supported by the results from a study that evaluated insulin signalling in type I and type II fibres isolated from the vastus lateralis in healthy lean people and people with obesity and insulin resistance [29]. It was found that compared with type II fibres, type I fibres have higher insulin receptor, GLUT4 and hexokinase protein contents, but both Akt2 protein content and insulin-stimulated AktThr308 phosphorylation, a measure of insulin action, were lower in type I than type II fibres, and the protein content and insulin-stimulated phosphorylation of other insulin-responsive signalling proteins were not different between type I and type II fibres [29].
Muscle perfusion is considered a major determinant of insulin-stimulated muscle glucose uptake because it regulates both insulin and glucose delivery to muscles [30, 31]. However, we did not detect an association between muscle perfusion and glucose uptake rates in individual muscle groups or all muscles combined in either the lean or obese groups or all participants combined. These findings confirm and extend the results from previous studies that found there was no association between insulin-stimulated thigh muscle perfusion and thigh muscle glucose uptake rates [32,33,34]. These data suggest that insulin delivery and glucose delivery to muscles are not rate limiting for insulin-stimulated glucose uptake and that local factors likely determine insulin action and glucose transport into myocytes. In fact, only small fractions of both insulin and glucose delivered to muscles are extracted by muscle ([32, 35,36,37] and present study), and defects in endothelial insulin transport, intracellular insulin signal transduction and intracellular glucose metabolism have been identified as key regulatory steps in determining muscle insulin availability and insulin-stimulated muscle glucose uptake [1, 3, 38,39,40,41,42,43,44].
We found that insulin-stimulated muscle glucose uptake accounted for ~60% of whole-body insulin-stimulated glucose disposal in both the lean and the obese groups, which is consistent with the results from other studies [32, 45, 46]. The difference between whole-body glucose disposal and muscle glucose uptake is presumably a function of glucose uptake in other organs, including intestine, heart, liver, kidneys, brain and adipose tissue [32, 47,48,49]. Nonetheless, we found that the whole-body glucose disposal rate, expressed in relation to FFM, correlated closely and almost along the line of identity with the mean muscle glucose uptake rate in both the lean and the obese groups, without a difference between groups. This close association between the two measurements is likely due to a similarity in the rate of insulin-stimulated glucose uptake in skeletal muscles and the rate of insulin-stimulated glucose uptake in other organs that constitute FFM, which are also affected by obesity-associated insulin resistance [32, 47,48,49]. In contrast, insulin-stimulated whole-body glucose disposal rate, expressed per kg body weight, markedly underestimated the mean muscle glucose uptake rate, particularly in people with obesity. This difference is likely related to the low rate of insulin-stimulated glucose disposal in adipose tissue and the small contribution of adipose tissue to total whole-body insulin-stimulated glucose disposal, even when adipose tissue mass is large [32, 45, 50]. Therefore, our data support the use of insulin-stimulated whole-body glucose disposal expressed in relation to FFM, but not in relation to total body weight, as a robust surrogate measure of muscle glucose uptake rate.
In summary, we found considerable heterogeneity in insulin-stimulated glucose uptake rates among different muscle groups in people that was not related to muscle perfusion or fibre type composition, presumably because local tissue and/or myocyte-specific factors, such as endothelial insulin transport, intracellular insulin signal transduction and glucose metabolism, regulate both insulin availability and action and glucose uptake. Furthermore, we found that the impairment in skeletal muscle insulin-stimulated glucose uptake in people with obesity is generalised across all major muscles, without specific defects in any one muscle group. Our data also demonstrate that although muscle accounts for only about two-thirds of whole-body glucose disposal rate, insulin-stimulated whole-body glucose uptake expressed per kg FFM provides a reliable index of insulin-stimulated muscle glucose uptake.
Data availability
The data generated during the current study are available from the corresponding author upon reasonable request. No resources were generated.
Abbreviations
- CT:
-
Computed tomography
- [18F]FDG:
-
[18F]fluorodeoxyglucose
- FFM:
-
Fat-free mass
- PET:
-
Positron emission tomography
References
Roden M, Shulman GI (2019) The integrative biology of type 2 diabetes. Nature 576(7785):51–60. https://doi.org/10.1038/s41586-019-1797-8
DeFronzo RA, Tripathy D (2009) Skeletal muscle insulin resistance is the primary defect in type 2 diabetes. Diabetes Care 32(Suppl 2):S157–S163. https://doi.org/10.2337/dc09-S302
Wasserman DH, Ayala JE (2005) Interaction of physiological mechanisms in control of muscle glucose uptake. Clin Exp Pharmacol Physiol 32(4):319–323. https://doi.org/10.1111/j.1440-1681.2005.04191.x
James DE, Jenkins AB, Kraegen EW (1985) Heterogeneity of insulin action in individual muscles in vivo: euglycemic clamp studies in rats. Am J Phys 248(5 Pt 1):E567–E574. https://doi.org/10.1152/ajpendo.1985.248.5.E567
Henriksen EJ, Bourey RE, Rodnick KJ, Koranyi L, Permutt MA, Holloszy JO (1990) Glucose transporter protein content and glucose transport capacity in rat skeletal muscles. Am J Phys 259(4 Pt 1):E593–E598
Megeney LA, Neufer PD, Dohm GL et al (1993) Effects of muscle activity and fiber composition on glucose transport and GLUT-4. Am J Phys 264(4 Pt 1):E583–E593. https://doi.org/10.1152/ajpendo.1993.264.4.E583
Pataky MW, Wang H, Yu CS et al (2017) High-fat diet-induced insulin resistance in single skeletal muscle fibers is fiber type selective. Sci Rep 7(1):13642. https://doi.org/10.1038/s41598-017-12682-z
Clerk LH, Rattigan S, Clark MG (2002) Lipid infusion impairs physiologic insulin-mediated capillary recruitment and muscle glucose uptake in vivo. Diabetes 51(4):1138–1145. https://doi.org/10.2337/diabetes.51.4.1138
Kraegen EW, James DE, Storlien LH, Burleigh KM, Chisholm DJ (1986) In vivo insulin resistance in individual peripheral tissues of the high fat fed rat: assessment by euglycaemic clamp plus deoxyglucose administration. Diabetologia 29(3):192–198. https://doi.org/10.1007/bf02427092
Conte C, Fabbrini E, Kars M, Mittendorfer B, Patterson BW, Klein S (2012) Multiorgan insulin sensitivity in lean and obese subjects. Diabetes Care 35(6):1316–1321. https://doi.org/10.2337/dc11-1951
Ter Horst KW, Serlie MJ (2020) Normalization of metabolic flux data during clamp studies in humans. Metabolism 104:154168. https://doi.org/10.1016/j.metabol.2020.154168
Phielix E, Begovatz P, Gancheva S et al (2019) Athletes feature greater rates of muscle glucose transport and glycogen synthesis during lipid infusion. JCI Insight 4(21):e127928. https://doi.org/10.1172/jci.insight.127928
Camastra S, Gastaldelli A, Mari A et al (2011) Early and longer term effects of gastric bypass surgery on tissue-specific insulin sensitivity and beta cell function in morbidly obese patients with and without type 2 diabetes. Diabetologia 54(8):2093–2102. https://doi.org/10.1007/s00125-011-2193-6
Tirrell TF, Cook MS, Carr JA, Lin E, Ward SR, Lieber RL (2012) Human skeletal muscle biochemical diversity. J Exp Biol 215(Pt 15):2551–2559. https://doi.org/10.1242/jeb.069385
Johnson MA, Polgar J, Weightman D, Appleton D (1973) Data on the distribution of fibre types in thirty-six human muscles. An autopsy study. J Neurol Sci 18:111–129
Smith GI, Yoshino J, Kelly SC et al (2016) High protein intake during weight loss therapy eliminates the weight loss-induced improvement in insulin action in postmenopausal women. Cell Rep 17(3):849–861. https://doi.org/10.1016/j.celrep.2016.09.047
Bryniarski AR, Meyer GA (2019) Brown Fat Promotes Muscle Growth During Regeneration. J Orthop Res 37(8):1817–1826. https://doi.org/10.1002/jor.24324
Muzik O, Mangner TJ, Leonard WR, Kumar A, Janisse J, Granneman JG (2013) 15O PET measurement of blood flow and oxygen consumption in cold-activated human brown fat. J Nucl Med 54(4):523–531. https://doi.org/10.2967/jnumed.112.111336
Kelley DE, Williams KV, Price JC, Goodpaster B (1999) Determination of the lumped constant for [18F] fluorodeoxyglucose in human skeletal muscle. J Nucl Med 40(11):1798–1804
Patlak CS, Blasberg RG (1985) Graphical evaluation of blood-to-brain transfer constants from multiple-time uptake data. Generalizations. J Cereb Blood Flow Metab 5(4):584–590. https://doi.org/10.1038/jcbfm.1985.87
Buckinx F, Landi F, Cesari M et al (2018) Pitfalls in the measurement of muscle mass: a need for a reference standard. J Cachexia Sarcopenia Muscle 9(2):269–278. https://doi.org/10.1002/jcsm.12268
Tanner CJ, Barakat HA, Dohm GL et al (2002) Muscle fiber type is associated with obesity and weight loss. Am J Physiol Endocrinol Metab 282(6):E1191–E1196. https://doi.org/10.1152/ajpendo.00416.2001
Lillioja S, Young AA, Culter CL et al (1987) Skeletal muscle capillary density and fiber type are possible determinants of in vivo insulin resistance in man. J Clin Invest 80(2):415–424. https://doi.org/10.1172/JCI113088
Nyholm B, Qu Z, Kaal A et al (1997) Evidence of an increased number of type IIb muscle fibers in insulin-resistant first-degree relatives of patients with NIDDM. Diabetes 46(11):1822–1828. https://doi.org/10.2337/diab.46.11.1822
Holmang A, Brzezinska Z, Bjorntorp P (1993) Effects of hyperinsulinemia on muscle fiber composition and capitalization in rats. Diabetes 42(7):1073–1081. https://doi.org/10.2337/diab.42.7.1073
Houmard JA, O’Neill DS, Zheng D, Hickey MS, Dohm GL (1999) Impact of hyperinsulinemia on myosin heavy chain gene regulation. J Appl Physiol (1985) 86(6):1828–1832. https://doi.org/10.1152/jappl.1999.86.6.1828
Sylow L, Kleinert M, Richter EA, Jensen TE (2017) Exercise-stimulated glucose uptake—regulation and implications for glycaemic control. Nat Rev Endocrinol 13(3):133–148. https://doi.org/10.1038/nrendo.2016.162
Krogh-Madsen R, Thyfault JP, Broholm C et al (2010) A 2-wk reduction of ambulatory activity attenuates peripheral insulin sensitivity. J Appl Physiol (1985) 108(5):1034–1040. https://doi.org/10.1152/japplphysiol.00977.2009
Albers PH, Pedersen AJ, Birk JB et al (2015) Human muscle fiber type-specific insulin signaling: impact of obesity and type 2 diabetes. Diabetes 64(2):485–497. https://doi.org/10.2337/db14-0590
Wasserman DH, Wang TJ, Brown NJ (2018) The vasculature in prediabetes. Circ Res 122(8):1135–1150. https://doi.org/10.1161/CIRCRESAHA.118.311912
Barrett EJ, Rattigan S (2012) Muscle perfusion: its measurement and role in metabolic regulation. Diabetes 61(11):2661–2668. https://doi.org/10.2337/db12-0271
Ferrannini E, Iozzo P, Virtanen KA, Honka MJ, Bucci M, Nuutila P (2018) Adipose tissue and skeletal muscle insulin-mediated glucose uptake in insulin resistance: role of blood flow and diabetes. Am J Clin Nutr 108(4):749–758. https://doi.org/10.1093/ajcn/nqy162
Utriainen T, Nuutila P, Takala T et al (1997) Intact insulin stimulation of skeletal muscle blood flow, its heterogeneity and redistribution, but not of glucose uptake in non-insulin-dependent diabetes mellitus. J Clin Invest 100(4):777–785. https://doi.org/10.1172/JCI119591
Raitakari M, Nuutila P, Ruotsalainen U et al (1996) Evidence for dissociation of insulin stimulation of blood flow and glucose uptake in human skeletal muscle: studies using [15O]H2O, [18F]fluoro-2-deoxy-D-glucose, and positron emission tomography. Diabetes 45(11):1471–1477. https://doi.org/10.2337/diab.45.11.1471
Williams KV, Price JC, Kelley DE (2001) Interactions of impaired glucose transport and phosphorylation in skeletal muscle insulin resistance: a dose-response assessment using positron emission tomography. Diabetes 50(9):2069–2079. https://doi.org/10.2337/diabetes.50.9.2069
Eggleston EM, Jahn LA, Barrett EJ (2007) Hyperinsulinemia rapidly increases human muscle microvascular perfusion but fails to increase muscle insulin clearance: evidence that a saturable process mediates muscle insulin uptake. Diabetes 56(12):2958–2963. https://doi.org/10.2337/db07-0670
van Raalte DH, van der Palen E, Idema P et al (2020) Peripheral Insulin Extraction in Non-Diabetic Subjects and Type 2 Diabetes Mellitus Patients. Exp Clin Endocrinol Diabetes 128(8):520–527. https://doi.org/10.1055/a-0808-4029
Bertoldo A, Pencek RR, Azuma K et al (2006) Interactions between delivery, transport, and phosphorylation of glucose in governing uptake into human skeletal muscle. Diabetes 55(11):3028–3037. https://doi.org/10.2337/db06-0762
Goodpaster BH, Bertoldo A, Ng JM et al (2014) Interactions among glucose delivery, transport, and phosphorylation that underlie skeletal muscle insulin resistance in obesity and type 2 Diabetes: studies with dynamic PET imaging. Diabetes 63(3):1058–1068. https://doi.org/10.2337/db13-1249
Williams IM, McClatchey PM, Bracy DP, Bonner JS, Valenzuela FA, Wasserman DH (2020) Transendothelial Insulin Transport is Impaired in Skeletal Muscle Capillaries of Obese Male Mice. Obesity (Silver Spring) 28(2):303–314. https://doi.org/10.1002/oby.22683
Sjostrand M, Gudbjornsdottir S, Holmang A, Lonn L, Strindberg L, Lonnroth P (2002) Delayed transcapillary transport of insulin to muscle interstitial fluid in obese subjects. Diabetes 51(9):2742–2748. https://doi.org/10.2337/diabetes.51.9.2742
Castillo C, Bogardus C, Bergman R, Thuillez P, Lillioja S (1994) Interstitial insulin concentrations determine glucose uptake rates but not insulin resistance in lean and obese men. J Clin Invest 93(1):10–16. https://doi.org/10.1172/JCI116932
Chiu JD, Richey JM, Harrison LN et al (2008) Direct administration of insulin into skeletal muscle reveals that the transport of insulin across the capillary endothelium limits the time course of insulin to activate glucose disposal. Diabetes 57(4):828–835. https://doi.org/10.2337/db07-1444
Kolterman OG, Insel J, Saekow M, Olefsky JM (1980) Mechanisms of insulin resistance in human obesity: evidence for receptor and postreceptor defects. J Clin Invest 65(6):1272–1284. https://doi.org/10.1172/JCI109790
Virtanen KA, Iozzo P, Hallsten K et al (2005) Increased fat mass compensates for insulin resistance in abdominal obesity and type 2 diabetes: a positron-emitting tomography study. Diabetes 54(9):2720–2726. https://doi.org/10.2337/diabetes.54.9.2720
Baron AD, Brechtel G, Wallace P, Edelman SV (1988) Rates and tissue sites of non-insulin- and insulin-mediated glucose uptake in humans. Am J Physiol 255(6 Pt 1):E769–E774
Boersma GJ, Johansson E, Pereira MJ et al (2018) Altered Glucose Uptake in Muscle, Visceral Adipose Tissue, and Brain Predict Whole-Body Insulin Resistance and may Contribute to the Development of Type 2 Diabetes: A Combined PET/MR Study. Horm Metab Res 50(8):627–639. https://doi.org/10.1055/a-0643-4739
Koffert JP, Mikkola K, Virtanen KA et al (2017) Metformin treatment significantly enhances intestinal glucose uptake in patients with type 2 diabetes: Results from a randomized clinical trial. Diabetes Res Clin Pract 131:208–216. https://doi.org/10.1016/j.diabres.2017.07.015
Honka H, Makinen J, Hannukainen JC et al (2013) Validation of [18F]fluorodeoxyglucose and positron emission tomography (PET) for the measurement of intestinal metabolism in pigs, and evidence of intestinal insulin resistance in patients with morbid obesity. Diabetologia 56(4):893–900. https://doi.org/10.1007/s00125-012-2825-5
Honka MJ, Latva-Rasku A, Bucci M et al (2018) Insulin-stimulated glucose uptake in skeletal muscle, adipose tissue and liver: a positron emission tomography study. Eur J Endocrinol 178(5):523–531. https://doi.org/10.1530/EJE-17-0882
Acknowledgements
The authors thank the staff of the Center for Human Nutrition, the Clinical Translational Research Unit, the Clinical Translational Imaging Unit and the Division of Radiological Sciences for help with participant recruitment, scheduling and testing and for technical assistance with sample processing and data analysis, and the study participants for their time and effort.
Authors’ relationships and activities
The authors declare that there are no relationships or activities that might bias, or be perceived to bias, their work.
Funding
The work presented in this manuscript was supported by National Institutes of Health grants R01 DK115400, P30 DK56341 (Nutrition Obesity Research Center), P30 DK020579 (Diabetes Research Center), P30 AR074992 (Musculoskeletal Research Center) and UL1TR000448 (Clinical Translational Science Award), and a grant from the American Diabetes Association (1-18-ICTS-119).
Author information
Authors and Affiliations
Contributions
BM and RJG designed the study. All authors contributed to data acquisition, data analysis and data interpretation. BM wrote the first draft of the manuscript. The co-authors contributed to the revision of the manuscript for crucial intellectual content. All authors approved the final version of the manuscript to be published. BM is the guarantor of this work, had full access to all the data in the study and assumes full responsibility for the integrity of the data and the accuracy of the data analysis.
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
ESM
(PDF 850 kb)
Rights and permissions
About this article
Cite this article
Koh, HC.E., van Vliet, S., Meyer, G.A. et al. Heterogeneity in insulin-stimulated glucose uptake among different muscle groups in healthy lean people and people with obesity. Diabetologia 64, 1158–1168 (2021). https://doi.org/10.1007/s00125-021-05383-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00125-021-05383-w