Abstract
Aims/hypothesis
Given the importance of glucagon in the development of type 2 diabetes and as a potential therapeutic agent, the aim of this study was to characterise glucagon kinetics in mice and its regulation by the nutritional state.
Methods
Anaesthetised C57BL/6 mice fed normal or high-fat diets, or fasted, were injected intravenously with glucagon (0.1, 0.3, 1.0, 10.0 or 20 μg/kg); blood samples were withdrawn before injection and 1, 3, 5, 10, 20 min thereafter for glucagon assay by RIA. Glucagon kinetics were described by two-compartment models using a population analysis.
Results
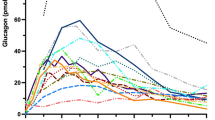
The population mean and between-animal SD of glucagon clearance in the fed mice was 6.03 ± 2.58 ml/min, with a rapid elimination half-life of 2.92 ± 1.21 min. Fasted mice showed a slower glucagon clearance. The kinetics of glucagon in the fed and fasted group was linear across this large dose range. The mice fed a high-fat diet, however, showed non-linear kinetics with a faster terminal clearance of 20.4 ± 5.45 ml/min (p < 0.001) and a shorter elimination half-life of 1.59 ± 0.606 (p < 0.001) min relative to normal mice.
Conclusions/interpretation
This first systematic dose-ranging study of glucagon kinetics produced several findings: (1) a linear two-compartment model describes glucagon in normal C57BL/6 mice; (2) fasting reduces the clearance of glucagon and (3) high-fat diet enhances the clearance of glucagon. These results may direct future studies on glucagon physiology and indicate that there are other mechanisms, not included in the current model, needed to fully explain glucagon’s kinetics.



Similar content being viewed by others
Abbreviations
- DPP-4:
-
Dipeptidyl peptidase-4
References
Dunning BE, Foley JE, Ahrén B (2005) Alpha cell function in health and disease: influence of glucagon-like peptide-1. Diabetologia 48:1700–1713
Dunning BE, Gerich JE (2007) The role of alpha-cell dysregulation in fasting and postprandial hyperglycemia in typ 2 diabetes and therapeutic implications. Endocr Rev 28:253–283
Roden M, Perseghin G, Petersen KF et al (1996) The roles of insulin and glucagon in the regulation of hepatic glycogen synthesis and turnover in humans. J Clin Invest 97:642–648
Jiang G, Zhang BB (2003) Glucagon and regulation of glucose metabolism. Am J Physiol Endocrinol Metab 284:E671–E678
Liljenquist JE, Mueller GL, Cherrington AD (1977) Evidence for an important role of glucagon in the regulation of hepatic glucose production in normal man. J Clin Invest 59:369–374
Bagger JI, Knop FK, Holst JJ, Vilsboll T (2011) Glucagon antagonism as a potential therapeutic target in type 2 diabetes. Diabetes Obes Metab 13:965–971
Cho YM, Merchant CE, Kieffer TJ (2012) Targeting the glucagon receptor family for diabetes and obesity therapy. Pharmacol Ther 135:247–278
Habegger KM, Heppner KM, Geary N, Bartness TJ, DiMarchi R, Tschöp MH (2010) The metabolic actions of glucagon revisited. Nat Rev Endocrinol 6:689–697
Dobbins RL, Davis SN, Neal DW, Cobelli C, Jaspan J, Cherrington AD (1995) Compartment modeling of glucagon kinetics in the conscious dog. Metab Clin Exp 44:452–459
Deacon CF, Kelstrup M, Trebbien R, Klarskov L, Olesen M, Holst JJ (2003) Differential regional metabolism of glucagon in anesthetized pigs. Am J Physiol Endocrinol Metab 285:552–560
Lefebvre P, Luyckx A (1974) Renal handling of endogenous glucagon in the dog: comparison with insulin. Metabolism 23:753–761
Authier F, Desbuquois B (2008) Glucagon receptors. Cell Mol Life Sci 65:1880–1899
Davidian M, Giltinan DM (1995) Nonlinear models for repeated measurement data. Chapman and Hall, London
D’Argenio DZ, Schumitzky A, Wang X (2009) ADAPT 5 user’s guide: pharmacokinetic/pharmacodynamic systems analysis software. Biomedical Simulations Resource, Los Angeles
Day JW, Gelfanov V, Smiley D et al (2012) Optimization of co-agonism at GLP-1 and glucagon receptors to safely maximize weight reduction in DIO-rodents. Biopolymers 98:443–450
Ahrén B, Landin-Olsson M, Jansson PA, Svensson M, Holmes D, Schweizer A (2004) Inhibition of dipeptidyl peptidase-4 reduces glycemia, sustains insulin levels and reduces glucagon levels in type 2 diabetes. J Clin Endocrinol Metab 89:2078–2084
Ruiter M, La Fleur SE, van Heijningen C, van der Vliet J, Kalsbeek A, Buijs RM (2003) The daily rhythm in plasma glucagon concentrations in the rat is modulated by the biological clock and by feeding behavior. Diabetes 52:1709–1715
Jaspan JB, Polonsky KS, Lewis M et al (1981) Hepatic metabolism of glucagon in the dog: contribution of the liver to overall metabolic disposal of glucagon. Am J Physiol Endocrinol Metab 240:233–244
Kervran A, Dubrasquet M, Blache P, Martinez J, Bataille D (1990) Metabolic clearance rates of oxyntomodulin and glucagon in the rat: contribution of the kidney. Regul Pept 31:41–52
Acknowledgements
This works was presented in part in abstract form at the American Diabetes Association’s 73rd Scientific Sessions, 2013, Chicago, IL, USA. We are grateful to K. Andersson (Department of Clinical Sciences, Lund University) for her technical assistance in the conduct of the experiments.
Funding
This work was supported by Women in Science and Engineering Undergraduate Research Fellowship from Univ. of Southern California to AZ (DZD mentor), National Institutes of Health grant P41-EB001978 to DZD and Swedish Research Council (Grant no. 6834), Region Skåne and Faculty of Medicine, Lund University to BA.
Duality of interest
The authors declare that there is no duality of interest associated with this manuscript.
Contribution statement
AZ and DZD performed data analysis and modelling, and contributed to interpretation of results and writing the paper. GP participated in the design of the study, in the interpretation of the results and in the revision of the manuscript. BA designed the study, performed the experiments and contributed to interpretation of results and writing the paper. All authors approved the final version of the manuscript to be published.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM Methods
(PDF 36.9 kb)
Rights and permissions
About this article
Cite this article
Zhou, A., Pacini, G., Ahrén, B. et al. Glucagon clearance is regulated by nutritional state: evidence from experimental studies in mice. Diabetologia 57, 801–808 (2014). https://doi.org/10.1007/s00125-013-3148-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00125-013-3148-x