Abstract
Aims/hypothesis
Sterol regulatory element binding protein-1c (SREBP-1c) is a master regulator of fatty acid synthase and controls lipogenesis. IRS-1 is the key insulin signalling mediator in skeletal muscle. In the present study, we investigated the role of SREBP-1c in the regulation of IRS-1 in skeletal muscle cells.
Methods
L6 muscle cells were treated with palmitic acid (PA) or metformin. Adenovirus vectors expressing Srebp-1c (also known as Srebf1) and small interfering RNA (siRNA) against Srebp-1c were transfected into the L6 cells. Protein–DNA interactions were assessed by luciferase reporter analysis, electrophoretic mobility shift assay and chromatin immunoprecipitation assay.
Results
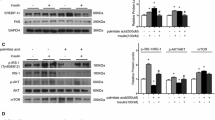
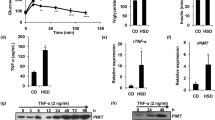
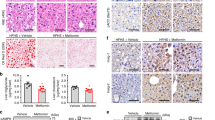
We found that both gene and protein expression of SREBP-1c was increased in contrast to IRS-1 expression in PA-treated L6 cells. SREBP-1c overproduction decreased Irs-1 mRNA and IRS-1 protein expression in a dose-dependent manner, and suppressed the resultant insulin signalling, whereas SERBP-1c knockdown by Serbp-1c siRNA blocked the downregulation of IRS-1 induced by PA. Protein–DNA interaction studies demonstrated that SREBP-1c was able to bind to the rat Irs-1 promoter region, thereby repressing its gene transcription. Of particular importance, we found that metformin treatment downregulated Srebp-1c promoter activity, decreased the specific binding of SREBP-1c to Irs-1 promoter and upregulated Irs-1 promoter activity in PA-cultured L6 cells.
Conclusions/interpretation
Our data indicate for the first time that SREBP-1c activation participates in skeletal muscle insulin resistance through a direct effect of suppressing Irs-1 transcription. These findings imply that SREBP-1c could serve as an attractive therapeutic target for insulin resistance.






Similar content being viewed by others
Abbreviations
- 2-NBDG:
-
2-[N-(7-nitrobenz-2-oxa-1,3-diazol-4-yl)-amino]-2-deoxy-d-glucose
- AMPK:
-
AMP-activated protein kinase
- bHLH:
-
Basic helix-loop-helix
- ChIP:
-
Chromatin immunoprecipitation assay
- EMSA:
-
Electrophoretic mobility shift assay
- FAS:
-
Fatty acid synthase
- GAPDH:
-
Glyceraldehyde-3-phosphate dehydrogenase
- GFP:
-
Green fluorescent protein
- LXR:
-
Liver X receptor
- PA:
-
Palmitic acid
- PI3K:
-
Phosphatidlyinositol-3-kinase
- SD rat:
-
Sprague-Dawley rat
- siRNA:
-
Small interfering RNA
- SRE:
-
Sterol regulatory element
- SREBP-1c:
-
Sterol regulatory element binding protein-1c
References
Unger RH (1995) Lipotoxicity in the pathogenesis of obesity-dependent NIDDM. Genetic and clinical implications. Diabetes 44:863–870
McGarry JD (2002) Banting lecture 2001: dysregulation of fatty acid metabolism in the etiology of type 2 diabetes. Diabetes 51:7–18
Petersen KF, Shulman GI (2006) Etiology of insulin resistance. Am J Med 119:S10–S16
Guillet-Deniau I, Mieulet V, Le Lay S et al (2002) Sterol regulatory element binding protein-1c expression and action in rat muscles: insulin-like effects on the control of glycolytic and lipogenic enzymes and UCP3 gene expression. Diabetes 51:1722–1728
Raghow R, Yellaturu C, Deng X, Park EA, Elam MB (2008) SREBPs: the crossroads of physiological and pathological lipid homeostasis. Trends Endocrinol Metab 19:65–73
Shimano H (2007) SREBP-1c and TFE3, energy transcription factors that regulate hepatic insulin signaling. J Mol Med 85:437–444
Ferré P, Foufelle F (2007) SREBP-1c transcription factor and lipid homeostasis: clinical perspective. Horm Res 68:72–82
Sesti G, Federici M, Hribal ML et al (2001) Defects of the insulin receptor substrate (IRS) system in human metabolic disorders. FASEB J 15:2099–2111
Kerouz NJ, Hörsch D, Pons S, Kahn CR (1997) Differential regulation of insulin receptor substrates-1 and -2 (IRS-1 and IRS-2) and phosphatidylinositol 3-kinase isoforms in liver and muscle of the obese diabetic (ob/ob) mouse. J Clin Invest 100:3164–3172
Huang C, Thirone AC, Huang X, Klip A (2005) Differential contribution of insulin receptor substrates 1 versus 2 to insulin signaling and glucose uptake in L6 myotubes. J Biol Chem 280:19426–19435
Bouzakri K, Zachrisson A, Al-Khalili L et al (2006) siRNA-base gene silencing reveals specialized roles of IRS-1/Akt2 and IRS-2/Akt1 in glucose and lipid metabolism in human skeletal muscle. Cell Metab 4:89–96
Taniguchi CM, Ueki K, Kahn R (2005) Complementary roles of IRS-1 and IRS-2 in the hepatic regulation of metabolism. J Clin Invest 115:718–727
Shimomura I, Matsuda M, Hammer RE et al (2000) Decreased IRS-2 and increased SREBP-1c lead to mixed insulin resistance and sensitivity in livers of lipodystrophic and ob/ob mice. Mol Cell 6:77–86
Tobe K, Suzuki R, Aoyama M et al (2001) Increased expression of the sterol regulatory element-binding protein-1 gene in insulin receptor substrate-2(−/−) mouse liver. J Biol Chem 276:38337–38340
Ide T, Shimano H, Yahagi N et al (2004) SREBP-1c suppress IRS-2-mediated insulin signalling in the liver. Nat Cell Biol 6:351–357
Bi Y, Cai MY, Liang H et al (2009) Increased CPT-1 expression and decreased SREBP-1c expression is associated with reduced intramuscular triglyceride accumulation after insulin therapy in high fat diet and streptozotocin induced diabetic rats. Metabolism 58:779–786
Bi Y, Sun WP, Chen X et al (2008) Effects of early insulin therapy on nuclear factor κB and cytokine gene expressions in the liver and muscle of fat-fed, streptozocin-treated diabetic SD rats. Acta Diabetol 45:167–178
Rachek LI, Musiyenko SI, LeDoux SP, Wilson GL (2007) Palmitate induced mitochondrial deoxyribonucleic acid damage and apoptosis in L6 rat skeletal muscle cells. Endocrinology 148:293–299
Logette E, Solary E, Corcos L (2005) Identification of a functional DNA binding site for the SREBP-1c transcription factor in the first intron of the human caspase-2 gene. Biochim Biophys Acta 1738:1–5
Salvadó L, Coll T, Gómez-Foix AM et al (2013) Oleate prevents saturated-fatty-acid-induced ER stress, inflammation and insulin resistance in skeletal muscle cells through an AMPK-dependent mechanism. Diabetologia 56:1372–1382
Kamei Y, Miura S, Suganami T et al (2008) Regulation of SREBP-1c gene expression in skeletal muscle: role of retinoid X receptor/liver X receptor and forkhead-O1 transcription factor. Endocrinology 149:2293–2305
Savage DB, Petersen KF, Shulman GI (2005) Mechanisms of insulin resistance in humans and possible links with inflammation. Hypertension 45:828–833
Houstis N, Rosen ED, Lander ES (2006) Reactive oxygen species have a causal role in multiple forms of insulin resistance. Nature 440:944–948
Hotamisligil GS (2005) Role of endoplasmic reticulum stress and c-Jun NH2-terminal kinase pathways in inflammation and origin of obesity and diabetes. Diabetes 54:S73–S78
Wellen KE, Hotamisligil GS (2005) Inflammation, stress, and diabetes. J Clin Invest 115:1111–1119
Hommelberg PP, Plat J, Sparks LM et al (2011) Palmitate-induced skeletal muscle insulin resistance does not require NF-κB activation. Cell Mol Life Sci 68:1215–1225
Kraegen EW, Cooney GJ (2008) Free fatty acids and skeletal muscle insulin resistance. Curr Opin Lipidol 19:235–241
Shao W, Espenshade PJ (2012) Expanding roles for SREBP in metabolism. Cell Metab 16:414–419
DeFronzo RA, Tripathy D (2009) Skeletal muscle insulin resistance is the primary defect in type 2 diabetes. Diabetes Care 32:S157–S163
Samuel VT, Shulman GI (2012) Mechanisms for insulin resistance: common threads and missing links. Cell 148:852–871
Slawik M, Vidal-Puig AJ (2006) Lipotoxicity, overnutrition and energy metabolism in aging. Ageing Res Rev 5:144–164
Guillet-Deniau I, Pichard AL, Kone A et al (2004) Glucose induces de novo lipogenesis in rat muscle satellite cells through a sterol-regulatory-element-binding-protein-1c-dependent pathway. J Cell Sci 117:1937–1944
Xie X, Liao H, Dang H et al (2009) Down-regulation of hepatic HNF4alpha gene expression during hyperinsulinemia via SREBPs. Mol Endocrinol 23:434–443
Chakravarty K, Wu SY, Chiang CM, Samols D, Hanson RW (2004) SREBP-1c and Sp1 interact to regulate transcription of the gene for phosphoenolpyruvate carboxykinase (GTP) in the liver. J Biol Chem 279:15385–15395
Okuno A, Tanaka J, Takahashi K et al (2009) Metformin primarily decreases plasma glucose not by gluconeogenesis suppression but by activating glucose utilization in a non-obese type 2 diabetes Goto-Kakizaki rats. Eur J Pharmacol 623:141–147
Zhou G, Myers R, Li Y et al (2001) Role of AMP-activated protein kinase in mechanism of metformin action. J Clin Invest 108:1167–1174
Li Y, Xu S, Mihaylova MM et al (2011) AMPK phosphorylates and inhibits SREBP activity to attenuate hepatic steatosis and atherosclerosis in diet-induced insulin-resistant mice. Cell Metab 13:376–388
Acknowledgements
We would like to thank Prof. Li Xiaoying from Shanghai Ruijin Hospital for his constructive discussion during manuscript preparation. Some of the data were presented as an abstract at the 49th Annual European Association for the Study of Diabetes (EASD) Meeting in 2013 (number 2194).
Funding
This work was sponsored by grants from the National Natural Science Foundation of China Grant Award (81270906, 30800539), 973 project (2012CB517506), National Science Fund for Distinguished Young Scholars (81025005), China postdoctoral Science Foundation (2012M521050), Jiangsu postdoctoral Science Foundation, Jiangsu Province’s Key Provincial Talents Program (RC2011011), Jiangsu Province’s Key Discipline of Medicine (XK201105), the Key Project of Nanjing Medical Science and Technology Development Foundation (ZKX11017), National Natural Science Foundation of China Grant Award (81000338, 81070636), New Drug Development, Construction and management of Clinical Biobank for Major Disease (2011ZX0907-001-08), the Project of National Key Clinical Division, Jiangsu Natural Science Foundation (KA037) and Guangdong Natural Science Foundation (10151008901000033).
Contribution statement
YB contributed to the study design, data interpretation, drafting the article, and final approval of the version to be published. WW contributed to the acquisition of data, drafting the article and approval of the final version. JS contributed to the study design, acquisition of data, drafting the article and approval of the final version. HL contributed the study design, data analysis, drafting the article and approval of the final version. WY, YC, ST, SC, MC, and SS contributed to acquisition of data, drafting the article and approval of the final version. QG contributed to the acquisition of data, drafting the article and approval of the final version. DZ contributed to the study design, acquisition of data, revision of the manuscript and final approval of the version to be published. JW contributed to the study design, acquisition of data, revision of the manuscript and final approval of the version to be published.
Duality of interest
The authors declare that there is no duality of interest associated with this manuscript.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Yan Bi, Wenjun Wu, Junfeng Shi and Hua Liang contributed equally to this study.
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM Table 1
(PDF 54 kb)
ESM Table 2
(PDF 8 kb)
ESM Table 3
(PDF 27 kb)
ESM Fig. 1
(PDF 104 kb)
ESM Fig. 2
(PDF 184 kb)
Rights and permissions
About this article
Cite this article
Bi, Y., Wu, W., Shi, J. et al. Role for sterol regulatory element binding protein-1c activation in mediating skeletal muscle insulin resistance via repression of rat insulin receptor substrate-1 transcription. Diabetologia 57, 592–602 (2014). https://doi.org/10.1007/s00125-013-3136-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00125-013-3136-1