Abstract
Aims/hypothesis
This study evaluated whether repeated non-attendance for diabetic eye screening is associated with the risk of sight-threatening diabetic retinopathy (STDR).
Methods
This was a cohort study of 6,556 residents with diabetes who were invited for screening between 2008 and 2011 in a population-based eye screening programme in inner London and who attended for their first-ever screen in 2008. The proportion of participants with STDR was evaluated in relation to the number of years in which screening was missed.
Results
The proportion of participants who did not attend screening decreased between 2009 and 2011 (annual reduction 1.6% [95% CI 0.9%, 2.3%]). The adjusted relative odds of STDR for 210 participants who did not attend two consecutive years of screening were 3.76 (95% CI 2.14, 6.61; p < 0.001), compared with participants who were screened annually. In 605 participants with mild non-proliferative retinopathy at the first screen, the adjusted relative odds of developing proliferative or moderate to severe non-proliferative retinopathy were 5.72 (95% CI 7.43, 22.83; p = 0.013) for 53 participants who missed two screens.
Conclusions/interpretation
Patients who do not attend diabetic eye screening are at increased risk of developing STDR. Tracing of non-attenders with evidence of established retinopathy should be an important fail-safe procedure.
Similar content being viewed by others
Introduction
Diabetic retinopathy and maculopathy are complications of diabetes mellitus which can lead to visual loss if they are not detected and treated early. It is estimated that 5% of blindness worldwide is attributable to diabetic retinopathy, rising to 17% in high-income Western European countries [1]. Laser photocoagulation can reduce blindness resulting from sight-threatening diabetic retinopathy (STDR) if detected at an early stage [2, 3]. A number of Western European countries have organised national diabetic eye screening programmes. In England, a population-based diabetic eye screening programme was introduced in 2003 and rolled out between 2003 and 2008 [4]. All individuals registered in primary care with diabetes mellitus aged 12 or older are invited for screening through a call–recall system. Screening is by digital retinal photography. Patients with referable eye disease, including moderate or severe non-proliferative or proliferative retinopathy or referable maculopathy, are referred for further ophthalmology care.
Diabetic eye screening is performed annually but ongoing research is investigating the use of more flexible scheduling. The population eligible for screening has risen with the increase in the prevalence of known diabetes. The cost-effectiveness of diabetic eye screening programmes is in part affected by compliance with screening invitations [5], and non-attendance is costly [6]. Around 79% of individuals invited for diabetic eye screening in England took up the offer of screening in 2010/2011 [4]. Patients who do not attend for screening have poorer HbA1c and blood pressure control [7] and have been diagnosed with diabetes for longer [7, 8], all of which are risk factors for developing retinopathy [8–15]. These observations suggest that patients who do not attend for eye screening might be at increased risk of diabetic eye disease.
A randomised controlled trial design would be needed to provide evidence to recommend a change to the frequency of screening intervals. It is, however, possible in an observational study, using routinely collected screening data, to evaluate the impact on patients of not attending annual screening checks. We aimed to test the hypothesis that non-attendance at diabetic eye screening appointments may be associated with an increased incidence of STDR. We also aimed to describe uptake over time. A population-based cohort study was implemented based on the electronic records of the eye screening programme.
Methods
Design
A retrospective, population-based cohort study included all patients invited for screening at an inner London diabetic eye screening service. The study was approved as a service evaluation by the National Health Service (NHS) Research and Development Office for South London. The data were fully anonymised and informed consent from individual participants was not required.
Participants and eligibility criteria
The study population comprised all individuals who had first attended for diabetic eye screening in 2008 and who were resident in the London boroughs of Lambeth, Southwark or Lewisham. These three inner London boroughs have an estimated combined resident population of 867,300 persons. All patients aged 12 or older who are registered with diabetes in primary care are invited for annual eye screening.
We initially had 230,961 appointments for 45,295 participants for the complete years in which the screening programme was more fully implemented (1 January 2008 to 31 December 2011) for participants resident in Lambeth, Southwark or Lewisham (Fig. 1). We only analysed data for participants who first attended for screening in 2008 and excluded duplicate or undated appointments and participants who were ineligible for screening (for example, because they had no perception of light in either eye). Appointments and/or screening episodes for participants were not included if they had STDR detected at a previous screen. The final sample included 31,887 appointments for 6,556 participants.
We conducted three analyses. First, all participants were included in an analysis of the uptake of screening. Second, we evaluated the risk of STDR in relation to the number of years in which screening was missed. Participants were included if they had their first screening record in 2008 and if they either attended every subsequent annual screen or if they missed at least one subsequent annual screen before re-attending. We excluded participants who had an ‘unassessable’ screening result, had STDR detected at their first screen, or who only attended a first screen (Fig. 1). Finally, we described the sociodemographic characteristics of participants who had missed years of screening before re-attending and analysed whether these characteristics were associated with the number of years in which screening was missed.
Variables analysed
Appointment records were coded as either ‘attended’ if there was a corresponding screen result or ‘not attended’. Participants were considered to have STDR detected at screening if the result for either eye was graded as moderate or severe non-proliferative retinopathy (R2), proliferative retinopathy (R3) or referable maculopathy (M1) [16, 17].
The following variables were included in the analysis: age group, sex, general practitioner (GP)-recorded type of diabetes, self-reported ethnic origin, duration of diabetes (based on GP-recorded date of diagnosis), and index of multiple deprivation score divided into quintiles based on the distribution for England in 2010. The index of multiple deprivation score is an indicator of the level of social and material deprivation in small areas (comprising around 1,000 households). Participants are assigned scores linked to their postcode of residence.
Statistical analysis
We tabulated, for each year of study, the number of participants invited and the proportion not attending. Linear regression was used to examine changes in the number of participants invited between 2008 and 2011 (using one invitation per participant per year and clustering by participant); univariable generalised linear models were used to examine changes in the proportion of participants not attending for screening between 2009 and 2011 (with a binomial family and identity link and clustering by participant). We tabulated the proportion of participants who did not attend screening in one or two consecutive years and then attended who had referable retinopathy (moderate or severe non-proliferative retinopathy or proliferative retinopathy) and/or referable maculopathy and STDR. We used logistic regression to examine whether the risk of STDR related to the number of years in which screening was missed (adjusting for the screening year, duration of diabetes, age group, sex, index of multiple deprivation quintile and diabetes type). We tabulated participants’ sociodemographic characteristics grouped by the number of years in which screening was missed and used ordered logistic regression to model the relationships. We present the results of likelihood ratio tests used to examine the utility of each characteristic in independently explaining variance in this model. All analyses were conducted using Stata version 12.1 (StataCorp LP, College Station, TX, USA) [18]; p < 0.05 was accepted as a significant effect.
Results
Characteristics of the sample
In 2008, 6,556 participants attended for their first-ever screen. These participants had a total of 31,887 screening invitations between 2008 and 2011. The majority of participants had type 2 diabetes (92.4%) and 67.0% had been diagnosed with diabetes for fewer than 5 years. Most participants were of white ethnic origin (47.2%), followed by participants of Caribbean (17.8%) and African (12.5%) origin. Most participants were in the fourth (45.8%) or fifth (42.2%) most deprived quintile for England; 51.0% were male and the greatest proportion of participants were in the age group 65–74 years (23.4%).
Screening invitations and uptake over the period
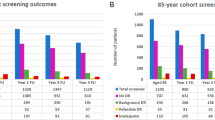
Most participants who attended for their first-ever screen in 2008 continued to be invited to attend in 2009, 2010 and 2011 (Table 1), and there was no significant decrease in the proportion invited (annual increment −461 participants per year [95% CI −1,016, 92]; Table 1). This was also the case for participants with type 1 diabetes (−30 participants per year [95% CI −79, 19]), type 2 diabetes (−403 participants per year [95% CI −870, 63]) and those whose type of diabetes was not recorded (−28 participants per year [95% CI −72, 16]). Data explaining the reasons why participants might not have been invited were incomplete. Among participants for whom there was a reason for their not being invited, the most common reasons were being under ophthalmology care (n = 515), being deceased (n = 447) and having moved out of the area (n = 140). Patients continue to be invited in this service even if they do not attend. Non-attendance at screening consistently decreased over the period (from 22.7% in 2009 to 19.5% in 2011 for all participants; Table 1). Non-attendance was greatest among participants whose type of diabetes was not recorded (range 38.1–50.7%) and lowest among those with type 2 diabetes (range 18.5–21.4%).
Risk of STDR and number of years in which screening was missed
We included 4,470 participants in the final analysis. Of the original 6,556 participants, 2,086 were excluded (Fig. 1). Reasons for exclusion included: the participant had an ‘unassessable’ screening result (n = 86); had STDR detected at the first screen (n = 105); or only attended a first screen (n = 1,895). Overall, 210 (4.7%) participants with previous screening results were not screened for two consecutive years and then re-attended, while 1,476 (33.0%) participants were not screened for 1 year and then re-attended (Table 2). Of participants who were not screened, 217 were not invited in 2009 and 244 were not invited in 2010. Participants who missed 1 year of screening had a median of 29 months (interquartile range 26–33) since their screen in 2008. The median was 33 months (interquartile range 29–36) for participants who missed 2 years of screening before re-attending.
Participants who were not screened for 2 years before attending for screening had 10.84 times higher odds of referable retinopathy being detected (95% CI 3.59, 32.70; Table 2) when compared with participants who were screened every year. There was no increased risk of referable retinopathy for participants who did not attend for screening in 1 year. The same pattern was evident for referable maculopathy and STDR, although the effect sizes were smaller. The relative odds of referable maculopathy when not screened for 2 years were 3.74 (95% CI 2.10, 6.65) and 3.76 (95% CI 2.14, 6.61) for STDR. There was evidence of a linear relationship between increasing number of years of screening missed and STDR (p < 0.001), referable retinopathy (p < 0.001) or referable maculopathy (p = 0.001).
Participants who had mild non-proliferative retinopathy detected at their first screen were more likely to have referable retinopathy or maculopathy or STDR detected at screening (Table 2). Among participants with no retinopathy at their first screen who were not screened for two consecutive years before attending, STDR was detected in 5.7% (OR 4.34 [95% CI 1.94, 9.71]). Among those who had mild retinopathy detected at their first screen and who were not screened for two consecutive years before attending, STDR was detected in 17.0% (OR 2.94 [95% CI 1.29, 6.72]). Since few participants who did not have retinopathy detected at their first screen had referable retinopathy detected at a subsequent screen (n = 8), logistic regression models (including exact logistic regression) could not be computed. There was evidence of a linear relationship between increasing years of screening missed and STDR (p = 0.005) or referable maculopathy (p = 0.011) for participants with no retinopathy at first screen and for STDR (p = 0.010) or referable maculopathy (p = 0.041) or referable retinopathy (p = 0.009) for participants with mild non-proliferative retinopathy at first screen.
The sociodemographic characteristics of the participants by years of screening missed before re-attending are shown in Table 3. Increasing duration of diabetes (p < 0.001), greater deprivation (p = 0.001) and type 1 diabetes (p < 0.001) were each associated with higher rates of missed screening. Missing screening was not associated with sex, but was more frequent in early adult years.
Discussion
Main findings
This study provides new information concerning the risk of STDR in relation to the number of years in which screening is missed. The results suggest that missing screening for as little as 2 years among participants who have mild retinopathy at their first screen may be associated with an increased likelihood of having referable retinopathy or maculopathy detected when they next return to be screened. Among participants without retinopathy detected at their first screen, the risk of STDR also increased with the number of years in which screening was missed, but the proportion with STDR was lower. There was a generally high level of uptake of screening, with non-attendance decreasing over time. Participants’ deprivation, duration of diabetes, age and type of diabetes were associated with missed screening, but missing screening was not restricted to any particular sociodemographic group. The greatest proportion of participants who missed 2 years of screening was in the age group 18–34 years. We think this is likely to reflect the highly mobile young population resident in the three boroughs under investigation. Participants might have missed screening either because they did not attend or because they were not invited.
Strengths and limitations of the study
The longitudinal nature of the data allowed us to examine the effect of non-attendance on the risk of STDR over time and account for participants’ previous screening results in our analysis. The data included all participants new to the programme in 2008 who were invited and attended for screening over a 4 year period in a population-based screening service. For this reason the findings are likely to reflect what is occurring in routine clinical practice at least in this area of London. As the screening service is based in three deprived and ethnically heterogeneous south London boroughs, it may not be representative of the English screening population. Non-attendance may in part be explained by list inflation, although this is likely to have reduced following a national initiative to remove duplicate patients from primary care records [19, 20]. Participants included in this study were a reduced group of the population invited for screening who had attended for screening in 2008. As most of these participants had been diagnosed with diabetes within the last 4 years, the results may be most applicable to patients who are new to screening. While it would be interesting to examine the risk of STDR and non-attendance among a population who were less recently diagnosed or among those who have never attended for screening, this was not the focus of the present analysis. We also only presented the demographic characteristics of a sample of participants from 2008. Although it is informative to look at the characteristics of participants who did not attend again over the period, we have previously presented this data elsewhere in a similar population [8].
Findings of other studies
The findings of the present study apply to individuals who fail to attend for screening; the same result may not be found if screening intervals are increased across the entire screening population. Patients who do not take care to attend for screening every year may also not attend to other aspects of their diabetes self-care, with possibly less satisfactory metabolic and blood pressure control. Clinical and organisational factors may also play a role in non-attendance and the outcomes of non-attendance, although we do not have the data to explore this here. A systematic review of interventions to improve uptake of diabetic eye screening found that the interventions that were effective increased patients’ and clinicians’ awareness of screening, improved access to healthcare, introduced computer-based registration or reminder systems, facilitated local collaborations between service providers, and developed community-based healthcare systems [21].
Some research has suggested that biennial screening may be suitable for some patients [22, 23]. Olafsdottir and Stefansson [22] reported that only participants with previously detected eye disease developed STDR within 2 years (although the sample was small; N = 296). Another study of 1,322 patients with type 2 diabetes did not observe any patients developing severe non-proliferative or proliferative retinopathy by 3 year follow-up if they had no retinopathy at baseline [24]. Misra et al [12], examining a larger sample, found that patients were no more likely to have referable retinopathy or maculopathy if they were screened every 18–24 months compared with every 12–18 months. Our results suggest that those patients who miss 1 or more years of screening represent a high-risk group with a greater chance of re-attending having developed retinopathy. Fail-safe procedures to trace non-attenders (including notifying patients’ GPs) are recommended by the English diabetic eye screening programme, although procedures are specific to each local service as there are no national guidelines.
We conclude that patients with previously detected retinopathy are at increasing risk of developing STDR if they do not attend for screening annually. Fail-safe procedures should include tracing of non-attenders who have evidence of established retinopathy.
Abbreviations
- GP:
-
General practitioner
- NHS:
-
National Health Service
- STDR:
-
Sight-threatening diabetic retinopathy
References
Resnikoff S, Pascolini D, Etya'ale D et al (2004) Global data on visual impairment in the year 2002. Bull World Health Organ 82:844–851
Early Treatment Diabetic Retinopathy Study Research Group (1985) Photocoagulation for diabetic macular edema. Early Treatment Diabetic Retinopathy Study report number 1. Arch Ophthalmol 103:1796–1806
Diabetic Retinopathy Study Group (1981) Photocoagulation treatment of proliferative diabetic retinopathy: relationship of adverse treatment effects to retinopathy severity. Diabetic Retinopathy Study report no. 5. Dev Ophthalmol 2:248–261
English National Diabetic Eye Screening Programme (2011) Annual report 2010/2011. English National Diabetic Eye Screening Programme, Gloucester
James M, Turner DA, Broadbent DM, Vora J, Harding SP (2000) Cost effectiveness analysis of screening for sight threatening diabetic eye disease. BMJ 320:1627–1631
Waqar S, Bullen G, Chant S, Salman R, Vaidya B, Ling R (2012) Cost implications, deprivation and geodemographic segmentation analysis of non-attenders (DNA) in an established diabetic retinopathy screening programme. Diabetes Metab Syndr 6:199–202
Leese GP, Boyle P, Feng Z, Emslie-Smith A, Ellis JD (2008) Screening uptake in a well-established diabetic retinopathy screening program: the role of geographical access and deprivation. Diabetes Care 31:2131–2135
Gulliford MC, Dodhia H, Chamley M et al (2010) Socio-economic and ethnic inequalities in diabetes retinal screening. Diabet Med 27:282–288
Klein R, Klein BE, Moss SE, Davis MD, DeMets DL (1984) The Wisconsin Epidemiologic Study of Diabetic Retinopathy. III. Prevalence and risk of diabetic retinopathy when age at diagnosis is 30 or more years. Arch Ophthalmol 102:527–532
Kohner EM, Aldington SJ, Stratton IM et al (1998) United Kingdom Prospective Diabetes Study, 30: diabetic retinopathy at diagnosis of non-insulin-dependent diabetes mellitus and associated risk factors. Arch Ophthalmol 116:297–303
Looker HC, Nyangoma SO, Cromie D et al (2012) Diabetic retinopathy at diagnosis of type 2 diabetes in Scotland. Diabetologia 55:2335–2342
Misra A, Bachmann MO, Greenwood RH et al (2009) Trends in yield and effects of screening intervals during 17 years of a large UK community-based diabetic retinopathy screening programme. Diabet Med 26:1040–1047
The Diabetes Control and Complications Trial Research Group (1993) The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med 329:977–986
Thomas RL, Dunstan F, Luzio SD et al (2012) Incidence of diabetic retinopathy in people with type 2 diabetes mellitus attending the Diabetic Retinopathy Screening Service for Wales: retrospective analysis. BMJ 344:e874
Younis N, Broadbent DM, Vora JP, Harding SP, The Liverpool Diabetic Eye Study Group (2003) Incidence of sight-threatening retinopathy in patients with type 2 diabetes in the Liverpool Diabetic Eye Study: a cohort study. Lancet 361:195–200
Early Treatment Diabetic Retinopathy Study Research Group (1991) Grading diabetic retinopathy from stereoscopic color fundus photographs—an extension of the modified Airlie House classification. ETDRS report number 10. Ophthalmology 98:786–806
NHS Diabetic Eye Screening Programme (2007) National screening programme for diabetic retinopathy workbook. NHS Diabetic Eye Screening Programme
Stata Corporation (2012) Stata statistical software. In. Stata Press, College Station, TX, USA
Department of Health (2004) GP Bulletin, 31
Ashworth M, Jenkins M, Burgess K et al (2005) Which general practices have higher list inflation? An exploratory study. Fam Pract 22:529–531
Zhang X, Norris SL, Saadine J et al (2007) Effectiveness of interventions to promote screening for diabetic retinopathy. Am J Prev Med 33:318–335
Olafsdottir E, Stefansson E (2007) Biennial eye screening in patients with diabetes without retinopathy: 10-year experience. Br J Ophthalmol 91:1599–1601
Jones CD, Greenwood RH, Misra A, Bachmann MO (2012) Incidence and progression of diabetic retinopathy during 17 years of a population-based screening program in England. Diabetes Care 35:592–596
Agardh E, Tababat-Khani P (2011) Adopting 3-year screening intervals for sight-threatening retinal vascular lesions in type 2 diabetic subjects without retinopathy. Diabetes Care 34:1318–1319
Acknowledgements
This work was supported by the National Institute for Health Research (NIHR) Biomedical Research Centre at Guy's and St Thomas' NHS Foundation Trust and King's College London. The views expressed are those of the authors and not necessarily those of the NHS, the NIHR or the Department of Health.
Funding
This research was funded by the South London Health Innovation and Education Cluster (HIEC).
Duality of interest
The authors have no non-financial interests that may be relevant to the submitted work. The authors declare that there is no duality of interest associated with this manuscript.
Contribution statement
All authors made a substantial contribution to the conception and design or analysis and interpretation of the data and gave final approval of the version to be published. ASF and MCG drafted the article and AF, HD, SS, SM, CC and ADC revised it critically for important intellectual content.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Forster, A.S., Forbes, A., Dodhia, H. et al. Non-attendance at diabetic eye screening and risk of sight-threatening diabetic retinopathy: a population-based cohort study. Diabetologia 56, 2187–2193 (2013). https://doi.org/10.1007/s00125-013-2975-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00125-013-2975-0