Abstract
Aims/hypothesis
Adaptor protein, phosphotyrosine interaction, pleckstrin homology domain and leucine zipper containing 1 (APPL1) is an adapter protein that positively mediates adiponectin signalling. Deficiency of APPL1 in the target tissues of insulin induces insulin resistance. We therefore aimed, in the present study, to determine its role in regulating pancreatic beta cell function.
Methods
A hyperglycaemic clamp test was performed to determine insulin secretion in APPL1 knockout (KO) mice. Glucose- and adiponectin-induced insulin release was measured in islets from APPL1 KO mice or INS-1(832/13) cells with either APPL1 knockdown or overproduction. RT-PCR and western blotting were conducted to analyse gene expression and protein abundance. Oxygen consumption rate (OCR), ATP production and mitochondrial membrane potential were assayed to evaluate mitochondrial function.
Results
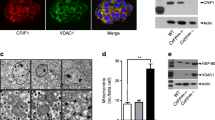
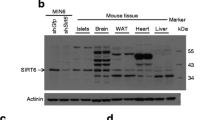
APPL1 is highly expressed in pancreatic islets, but its levels are decreased in mice fed a high-fat diet and db/db mice compared with controls. Deletion of the Appl1 gene leads to impairment of both the first and second phases of insulin secretion during hyperglycaemic clamp tests. In addition, glucose-stimulated insulin secretion (GSIS) is significantly decreased in islets from APPL1 KO mice. Conversely, overproduction of APPL1 leads to an increase in GSIS in beta cells. In addition, expression levels of several genes involved in insulin production, mitochondrial biogenesis and mitochondrial OCR, ATP production and mitochondrial membrane potential are reduced significantly in APPL1-knockdown beta cells. Moreover, suppression or overexproduction of APPL1 inhibits or stimulates adiponectin-potentiated GSIS in beta cells, respectively.
Conclusions/interpretation
Our study demonstrates the roles of APPL1 in regulating GSIS and mitochondrial function in pancreatic beta cells, which implicates APPL1 as a therapeutic target in the treatment of type 2 diabetes.








Similar content being viewed by others
Abbreviations
- Ad:
-
Adenovirus
- AMPK:
-
AMP activated protein kinase
- APPL1:
-
Adaptor protein, phosphotyrosine interaction, pleckstrin homology domain and leucine zipper containing 1
- EGFP:
-
Enhanced green fluorescent protein
- FCCP:
-
Carbonylcyanide p-trifluoromethoxyphenylhydrazone
- GIR:
-
Glucose infusion rate
- GSIS:
-
Glucose-stimulated insulin secretion
- GTT:
-
Glucose tolerance test
- HFD:
-
High-fat diet
- α-KIC:
-
α-Ketoisocaproate
- KO:
-
Knockout
- MMP:
-
Mitochondrial membrane potential
- MOI:
-
Multiplicity of infection
- OCR:
-
Oxygen consumption rate
- PGC-1α:
-
Peroxisome proliferator-activated receptor-γ coactivator-1α
- PH:
-
Pleckstrin homology
- ROS:
-
Reactive oxygen species
- SAME:
-
Succinic acid methyl ester
- sh:
-
Short hairpin
- TFAM:
-
Mitochondrial transcription factor A
- WT:
-
Wild-type
References
Weyer C, Bogardus C, Mott DM, Pratley RE (1999) The natural history of insulin secretory dysfunction and insulin resistance in the pathogenesis of type 2 diabetes mellitus. J Clin Invest 104:787–794
Karpe F, Dickmann JR, Frayn KN (2011) Fatty acids, obesity, and insulin resistance: time for a reevaluation. Diabetes 60:2441–2449
Mitsuuchi Y, Johnson SW, Sonoda G, Tanno S, Golemis EA, Testa JR (1999) Identification of a chromosome 3p14.3-21.1 gene, APPL, encoding an adaptor molecule that interacts with the oncoprotein-serine/threonine kinase AKT2. Oncogene 18:4891–4898
Deepa SS, Dong LQ (2009) APPL1: role in adiponectin signaling and beyond. Am J Physiol Endocrinol Metab 296:E22–E36
Mao X, Kikani CK, Riojas RA et al (2006) APPL1 binds to adiponectin receptors and mediates adiponectin signalling and function. Nat Cell Biol 8:516–523
Lee JR, Hahn HS, Kim YH et al (2011) Adaptor protein containing PH domain, PTB domain and leucine zipper (APPL1) regulates the protein level of EGFR by modulating its trafficking. Biochem Biophys Res Commun 415:206–211
Tian L, Luo N, Zhu X, Chung BH, Garvey WT, Fu Y (2012) Adiponectin-AdipoR1/2-APPL1 signaling axis suppresses human foam cell formation: differential ability of AdipoR1 and AdipoR2 to regulate inflammatory cytokine responses. Atherosclerosis 221:66–75
Chen YQ, Hsieh JT, Yao F et al (1999) Induction of apoptosis and G2/M cell cycle arrest by DCC. Oncogene 18:2747–2754
Wen L, Yang Y, Wang Y, Xu A, Wu D, Chen Y (2010) Appl1 is essential for the survival of Xenopus pancreas, duodenum, and stomach progenitor cells. Dev Dyn 239:2198–2207
Lin DC, Quevedo C, Brewer NE et al (2006) APPL1 associates with TrkA and GIPC1 and is required for nerve growth factor-mediated signal transduction. Mol Cell Biol 26:8928–8941
Cheng KK, Iglesias MA, Lam KS et al (2009) APPL1 potentiates insulin-mediated inhibition of hepatic glucose production and alleviates diabetes via Akt activation in mice. Cell Metab 9:417–427
Wang Y, Cheng KK, Lam KS et al (2011) APPL1 counteracts obesity-induced vascular insulin resistance and endothelial dysfunction by modulating the endothelial production of nitric oxide and endothelin-1 in mice. Diabetes 60:3044–3054
Holland WL, Miller RA, Wang ZV et al (2011) Receptor-mediated activation of ceramidase activity initiates the pleiotropic actions of adiponectin. Nat Med 17:55–63
Huypens P, Moens K, Heimberg H, Ling Z, Pipeleers D, van de Casteele M (2005) Adiponectin-mediated stimulation of AMP-activated protein kinase (AMPK) in pancreatic beta cells. Life Sci 77:1273–1282
Staiger K, Stefan N, Staiger H et al (2005) Adiponectin is functionally active in human islets but does not affect insulin secretory function or beta-cell lipoapoptosis. J Clin Endocrinol Metab 90:6707–6713
Wijesekara N, Krishnamurthy M, Bhattacharjee A, Suhail A, Sweeney G, Wheeler MB (2010) Adiponectin-induced ERK and Akt phosphorylation protects against pancreatic beta cell apoptosis and increases insulin gene expression and secretion. J Biol Chem 285:33623–33631
Chetboun M, Abitbol G, Rozenberg K et al (2012) Maintenance of redox state and pancreatic beta-cell function: role of leptin and adiponectin. J Cell Biochem 113:1966–1976
Muller D, Huang GC, Amiel S, Jones PM, Persaud SJ (2006) Identification of insulin signaling elements in human beta-cells: autocrine regulation of insulin gene expression. Diabetes 55:2835–2842
Cheng KK, Lam KS, Wu D et al (2012) APPL1 potentiates insulin secretion in pancreatic beta cells by enhancing protein kinase Akt-dependent expression of SNARE proteins in mice. Proc Natl Acad Sci U S A 109:8919–8924
Wang C, Mao R, van de Casteele M, Pipeleers D, Ling Z (2007) Glucagon-like peptide-1 stimulates GABA formation by pancreatic beta-cells at the level of glutamate decarboxylase. Am J Physiol Endocrinol Metab 292:E1201–E1206
Darling AJ, Boose JA, Spaltro J (1998) Virus assay methods: accuracy and validation. Biologicals 26:105–110
Hohmeier HE, Mulder H, Chen G, Henkel-Rieger R, Prentki M, Newgard CB (2000) Isolation of INS-1-derived cell lines with robust ATP-sensitive K+ channel-dependent and -independent glucose-stimulated insulin secretion. Diabetes 49:424–430
Xie Z, Ma X, Ji W et al (2010) Zbtb20 is essential for the specification of CA1 field identity in the developing hippocampus. Proc Natl Acad Sci U S A 107:6510–6515
Koyanagi M, Asahara S, Matsuda T et al (2011) Ablation of TSC2 enhances insulin secretion by increasing the number of mitochondria through activation of mTORC1. PLoS One 6:e23238
Wu M, Neilson A, Swift AL et al (2007) Multiparameter metabolic analysis reveals a close link between attenuated mitochondrial bioenergetic function and enhanced glycolysis dependency in human tumor cells. Am J Physiol Cell Physiol 292:C125–C136
Yano M, Watanabe K, Yamamoto T et al (2011) Mitochondrial dysfunction and increased reactive oxygen species impair insulin secretion in sphingomyelin synthase 1-null mice. J Biol Chem 286:3992–4002
Cleasby ME, Lau Q, Polkinghorne E et al (2011) The adaptor protein APPL1 increases glycogen accumulation in rat skeletal muscle through activation of the PI3-kinase signalling pathway. J Endocrinol 210:81–92
Saito T, Jones CC, Huang S, Czech MP, Pilch PF (2007) The interaction of Akt with APPL1 is required for insulin-stimulated Glut4 translocation. J Biol Chem 282:32280–32287
Marinho R, Ropelle ER, Cintra DE et al (2012) Endurance exercise training increases APPL1 expression and improves insulin signaling in the hepatic tissue of diet-induced obese mice, independently of weight loss. J Cell Physiol 227:2917–2926
Fang QC, Jia WP, Gao F et al (2008) Association of variants in APPL1 gene with body fat and its distribution in Chinese patients with type 2 diabetic mellitus. Zhonghua Yi Xue Za Zhi 88:369–373 [article in Chinese]
Leibowitz G, Khaldi MZ, Shauer A et al (2005) Mitochondrial regulation of insulin production in rat pancreatic islets. Diabetologia 48:1549–1559
MacDonald MJ (2007) Synergistic potent insulin release by combinations of weak secretagogues in pancreatic islets and INS-1 cells. J Biol Chem 282:6043–6052
Larsson NG, Wang J, Wilhelmsson H et al (1998) Mitochondrial transcription factor A is necessary for mtDNA maintenance and embryogenesis in mice. Nat Genet 18:231–236
Silva JP, Kohler M, Graff C et al (2000) Impaired insulin secretion and beta-cell loss in tissue-specific knockout mice with mitochondrial diabetes. Nat Genet 26:336–340
Ventura-Clapier R, Garnier A, Veksler V (2008) Transcriptional control of mitochondrial biogenesis: the central role of PGC-1alpha. Cardiovasc Res 79:208–217
Lin J, Wu PH, Tarr PT et al (2004) Defects in adaptive energy metabolism with CNS-linked hyperactivity in PGC-1alpha null mice. Cell 119:121–135
Leone TC, Lehman JJ, Finck BN et al (2005) PGC-1alpha deficiency causes multi-system energy metabolic derangements: muscle dysfunction, abnormal weight control and hepatic steatosis. PLoS Biol 3:e101
Maassen JA, ‘T Hart LM, van Essen E et al (2004) Mitochondrial diabetes: molecular mechanisms and clinical presentation. Diabetes 53(Suppl 1):S103–S109
Maechler P, Wollheim CB (2000) Mitochondrial signals in glucose-stimulated insulin secretion in the beta cell. J Physiol 529(Pt 1):49–56
Henquin JC (2000) Triggering and amplifying pathways of regulation of insulin secretion by glucose. Diabetes 49:1751–1760
Wang C, Xin X, Xiang R et al (2009) Yin-Yang regulation of adiponectin signaling by APPL isoforms in muscle cells. J Biol Chem 284:31608–31615
Qiao L, Kinney B, Yoo HS, Lee B, Schaack J, Shao J (2012) Adiponectin increases skeletal muscle mitochondrial biogenesis by suppressing mitogen-activated protein kinase phosphatase-1. Diabetes 61:1463–1470
Iwabu M, Yamauchi T, Okada-Iwabu M et al (2010) Adiponectin and AdipoR1 regulate PGC-1alpha and mitochondria by Ca(2+) and AMPK/SIRT1. Nature 464:1313–1319
Civitarese AE, Ukropcova B, Carling S et al (2006) Role of adiponectin in human skeletal muscle bioenergetics. Cell Metab 4:75–87
Fu A, Eberhard CE, Screaton RA (2013) Role of AMPK in pancreatic beta cell function. Mol Cell Endocrinol 366:127–134
Rutter GA, Leclerc I (2009) The AMP-regulated kinase family: enigmatic targets for diabetes therapy. Mol Cell Endocrinol 297:41–49
Rutter GA, Da Silva Xavier G, Leclerc I (2003) Roles of 5′-AMP-activated protein kinase (AMPK) in mammalian glucose homoeostasis. Biochem J 375:1–16
Sun G, Tarasov AI, McGinty J et al (2010) Ablation of AMP-activated protein kinase alpha1 and alpha2 from mouse pancreatic beta cells and RIP2.Cre neurons suppresses insulin release in vivo. Diabetologia 53:924–936
Acknowledgements
We thank Pengying Li and Kelei Dong (Department of Biochemistry and Molecular Biology, Shanghai Medical College of Fudan University, China) for technical help.
Funding
This work was supported by grants from the National Natural Science Foundation (30971121) (to CW), Shanghai Committee of Science and Technology (11140900900) (to CW), and National Institute of Health grant R01 DK080344 (to LQD).
Duality of interest
The authors declare that there is no duality of interest associated with this manuscript.
Contribution statement
CW and LQD were responsible for designing the experiments. XL, KM, LL, SW, YZ, MZ, JR, ZX, DS, WJZ were responsible for acquisition of data, and analysis and interpretation of data. CW, LQD and WJ analysed and interpreted data. CW drafted the manuscript. All authors critically revised and approved the final version of the manuscript.
Author information
Authors and Affiliations
Corresponding authors
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM Materials and methods
(PDF 16.0 kb)
ESM Table 1
(PDF 88.0 kb)
ESM Fig. 1
(PDF 18.4 kb)
ESM Fig. 2
(PDF 9.67 kb)
ESM Fig. 3
(PDF 10.7 kb)
ESM Fig. 4
(PDF 13.7 kb)
Rights and permissions
About this article
Cite this article
Wang, C., Li, X., Mu, K. et al. Deficiency of APPL1 in mice impairs glucose-stimulated insulin secretion through inhibition of pancreatic beta cell mitochondrial function. Diabetologia 56, 1999–2009 (2013). https://doi.org/10.1007/s00125-013-2971-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00125-013-2971-4