Abstract
Aims/hypothesis
Alterations in white adipose tissue (WAT) function, including changes in protein (adipokine) secretion and extracellular matrix (ECM) composition, promote an insulin-resistant state. We set out to identify novel adipokines regulated by body fat mass in human subcutaneous WAT with potential roles in adipose function.
Methods
Adipose transcriptome data and secretome profiles from conditions with increased/decreased WAT mass were combined. WAT donors were predominantly women. In vitro effects were assessed using recombinant protein. Results were confirmed by quantitative PCR/ELISA, metabolic assays and immunochemistry in human WAT and adipocytes.
Results
We identified a hitherto uncharacterised adipokine, semaphorin 3C (SEMA3C), the expression of which correlated significantly with body weight, insulin resistance (HOMA of insulin resistance [HOMAIR], and the rate constant for the insulin tolerance test [KITT]) and adipose tissue morphology (hypertrophy vs hyperplasia). SEMA3C was primarily found in mature adipocytes and had no direct effect on human adipocyte differentiation, lipolysis, glucose transport or the expression of β-oxidation genes. This could in part be explained by the significant downregulation of its cognate receptors during adipogenesis. In contrast, in pre-adipocytes, SEMA3C increased the production/secretion of several ECM components (fibronectin, elastin and collagen I) and matricellular factors (connective tissue growth factor, IL6 and transforming growth factor-β1). Furthermore, the expression of SEMA3C in human WAT correlated positively with the degree of fibrosis in WAT.
Conclusions/interpretation
SEMA3C is a novel adipokine regulated by weight changes. The correlation with WAT hypertrophy and fibrosis in vivo, as well as its effects on ECM production in human pre-adipocytes in vitro, together suggest that SEMA3C constitutes an adipocyte-derived paracrine signal that influences ECM composition and may play a pathophysiological role in human WAT.
Similar content being viewed by others
Introduction
White adipose tissue (WAT) is a highly plastic organ that can change considerably in size within and between individuals. Excess fat mass is associated with insulin resistance, type 2 diabetes and dyslipidaemia, and correlates with distinct changes in fat-cell size and adipose function, including altered lipid metabolism, increased interstitial fibrosis (due to elevated extracellular matrix [ECM] protein production and deposition) and a chronic pro-inflammatory state [1]. Studies assessing the effects of weight loss have demonstrated that upon reduction of fat mass to a non-obese state, most aspects of WAT function are normalised [2, 3].
To maintain a healthy metabolic profile during fat mass alterations, adaptive mechanisms involving signals between cells present within the tissue (e.g. adipocytes, adipocyte progenitor cells, immune cells and endothelial cells) are necessary. Results in recent years have demonstrated that adipocytes secrete a number of polypeptides (collectively termed adipokines), which, in humans, predominantly act locally through auto- or paracrine mechanisms. However, in obesity the production of several adipokines can be maladaptive and can promote an insulin-resistant state [4]. Although a number of human WAT/adipocyte secretome studies have been performed, the overlap between different studies is rather poor (see for instance [5–9]). At present, the total human adipokinome is estimated to contain well over 600 members but the list is still growing.
In this study, we set out to identify novel adipokines regulated by weight alterations in human subcutaneous WAT (scWAT) that could have an impact on WAT function. To this end, transcriptomic data obtained from scWAT of individuals with different forms of fat mass alterations (obesity, weight reduction by bariatric surgery and cancer cachexia) were combined with a secretome analysis performed in human WAT. This unbiased approach enabled us to identify a hitherto uncharacterised adipokine, semaphorin 3C (SEMA3C), which belongs to a group of secreted factors including seven individual gene members (SEMA3A–G). Very little is known regarding the role of SEMA3 members in WAT. Only one publication has studied the expression of Sema3a in rat WAT [10]. SEMA3s signal through cognate receptors and co-receptors termed plexins (PLXNs) and neuropilins (NRPs), respectively, but the intracellular pathways are poorly characterised [11]. We demonstrate that SEMA3C is primarily secreted from fat cells and that the expression in scWAT correlates with insulin resistance, fat-cell morphology and other measures of the metabolic syndrome. Functional studies in human adipocytes did not reveal any significant effects of SEMA3C. However, incubation with recombinant SEMA3C in human pre-adipocytes increased the expression and secretion of several ECM components and matricellular factors. In addition, expression of the gene encoding SEMA3C in human WAT correlated with interstitial fibrosis.
Methods
Clinical cohorts
The participants included in the cohorts are described, including relevant references, in the electronic supplementary material (ESM) Table 1. Clinical assessments were performed as described in the corresponding references. Abdominal scWAT was obtained by needle/surgical biopsies or as a waste product from cosmetic surgical procedures, as described previously [12]. For tissues used for cell culture experiments, there was no selection on the basis of age, sex or BMI. None of the participants were on any regular medication that might be expected to affect adipocyte function. Cachexia was defined as described in [13]. BMI was classified according to the WHO definition. The metabolic syndrome was defined according to recently described definitions [14] where waist circumference criteria from the International Diabetes Federation were used [15]. The project was conducted in accordance with the guidelines in The Declaration of Helsinki and the studies were approved by the Regional Ethics committee in Stockholm, Toulouse University Hospitals, the Third Faculty of Medicine in Prague and Hôtel-Dieu Hospital, Paris. Individual, written informed consent was obtained from all participants involved in the study.
Adipocyte isolation, cell culture and tissue fractionation
Mature adipocytes and stroma-vascular fraction (SVF) cells were isolated as described previously [16, 17]. Adipocyte morphology (i.e. the relative fat-cell size in relation to total fat mass) has been shown to correlate with insulin sensitivity and was determined as described [18].
Distinct cell populations of the SVF were isolated using an immunoselection/depletion protocol and cultured as described [16, 17, 19–21]. CD34+/CD31− cells were defined as progenitor cells, CD34+/CD31+ cells as endothelial cells, CD34−/CD14+ cells as macrophages and CD34−/CD14−/CD3+ as lymphocytes. For time course analysis, cells were lysed to obtain RNA at day 4/5, 8 and 12 after the induction of differentiation. For the secretome analysis, mature adipocytes and the various cell populations from the SVF were maintained separately ex vivo at 37°C in endothelial basal culture medium (0.1% BSA) for 24 h and their conditioned media were collected.
Transcriptome and secretome studies
For human transcriptome data, probesets identified to be differentially expressed, comparing obese with non-obese according to significance analysis of microarrays (SAM, false discovery rate 5%) [22], were used as a starting point. These probesets were extracted from the weight-loss studies and subsequently analysed with SAM. Thereafter, significantly regulated probesets corresponding to the same gene were averaged. This generated a set of genes that were regulated by obesity as well as by voluntary (energy restriction) and involuntary (cancer cachexia) weight loss. The list was filtered and genes with a low fold change (<15% comparing groups) were excluded, resulting in a list of 112 individual genes. Enrichment of predefined gene ontologies (GOs) were identified using Gene Ontology Tree Machine [23]. Messenger RNA levels of the class 3 SEMAs were separately extracted from human transcriptome profiling datasets and evaluated for differential expression in cohorts 1–3. Transcriptome analysis of different human subcutaneous WAT cell types was performed on cohort 4 as reported [24]. In the secretome analyses (cohort 5), media from each cell fraction were pooled. To optimise the detection of medium- and low-abundance proteins, depletion of highly abundant proteins from the media was performed using the ProteoMiner Protein Enrichment Kit (Bio-Rad, Hercules, CA, USA). Proteins were then separated on 1D SDS-PAGE gels (12% 12.5% constant concentration polyacrylamide slab gel; Bio-Rad). The resulting gel lanes were divided into slices (10/sample) before MS analysis. Proteins contained in gel slices were reduced, alkylated and digested with trypsin using standard in-gel digestion protocols. The digested peptides were then analysed using NanoLC/ESI LTQ-Orbitrap MS/MS (Thermo Fisher Scientific, Waltham, MA, USA), and the corresponding proteins were identified by automated database searching (Mascot Daemon, Matrix Science; http://mfpaq.sourceforge.net) against the Swiss-Prot/Trembl protein sequence database (http://www.uniprot.org). A comparative proteomic analysis of the proteins secreted by the different cell types contained in WAT (mature adipocytes, endothelial cells, macrophages, progenitor cells, endothelial cells and lymphocytes) led to the identification of 626 proteins (N. Viguerie, C.-I. Kolditz and D. Langin, unpublished data). To identify factors specifically produced by adipocytes, transcriptomic and proteomic data from the different cell types were compared. This list was cross-checked with the list of the 112 weight-regulated genes.
RNA isolation, cDNA synthesis and quantitative PCR
RNA isolation, cDNA synthesis and real-time PCR were performed exactly as described [12]. For SYBR Green assays, 5 ng of cDNA was mixed with 2× iQ SYBR Green Supermix (Eurogentec, Ougrée, Belgium) and primers (Invitrogen, Carlsbad, CA, USA) in a final volume of 25 μl. For TaqMan assays, 10 ng of cDNA was mixed with 2× TaqMan Universal PCR Master Mix and TaqMan primers (Applied Biosystems, Foster City, CA, USA) in a final volume of 20 μl. Messenger RNA levels were normalised to an internal reference—18S rRNA (intact WAT, SVF and mature adipocytes) or LRP10 mRNA (in vitro differentiated pre-adipocytes/adipocytes)—using a comparative Ct method calculated by the formula\( {{{{2^{{\varDelta {{\mathrm{C}}_{\mathrm{t}}}}}}^{{-\mathrm{target}\ \mathrm{gene}}}}} \left/ {{{2^{{\varDelta {{\mathrm{C}}_{\mathrm{t}}}}}}^{{-\mathrm{reference}\ \mathrm{gene}}}}} \right.} \). TaqMan assays and SYBR Green primers are listed in ESM Table 2.
ELISA
The level of SEMA3C secreted from intact scWAT (300 mg pieces in 3 ml medium) was assessed and incubated as described [25]. Medium was saved at −70°C for determination of SEMA3C levels according to the manufacturer’s instructions (E80919Hu; USCN Life Science, Wuhan, Peoples Republic of China). The standard curve of the SEMA3C ELISA kit ranged between 78 and 5,000 pg/ml and the lowest detectable level was 27.7 pg/ml, according to the instruction manual provided by the manufacturer. The specificity of the ELISA kit was confirmed by western blot using two different antibodies (see ESM Fig. 1 and ESM Methods; Santa Cruz Biotechnology, Santa Cruz, CA, USA and R&D Systems, Minneapolis, MN, USA, respectively). The secretion of SEMA3C protein from WAT explants of obese and non-obese participants was related to the number of fat cells in the incubated tissue sample. Connective tissue growth factor (CTGF, E90010Hu; USCN Life Science), ELN (E91337Hu; USCN Life Science), IL6 (D6050; R&D Systems) and transforming growth factor-β1 (TGF-β1) (DB100B; R&D Systems) protein secretion was measured in conditioned culture media from in vitro differentiated adipocytes according to the manufacturers’ instructions.
Immunofluorescence and confocal microscopy
Immunofluorescence analysis of collagen/fibronectin networks produced by human pre-adipocytes was performed as described previously [26] and as detailed in the ESM Methods.
Immunohistochemical analyses
Subcutaneous WAT samples were prepared for immunohistochemical analyses as described in the ESM Methods and elsewhere [27, 28]. Representative microphotographs are presented in ESM Figs 2, 3.
Hypoxia experiments
Conditioned media from mature adipocytes were obtained after 24 h culture in endothelial cell basal medium (1/3, vol./vol.) supplemented with 0.1% BSA, 100 U/ml penicillin and 100 g/ml streptomycin in CLINIcell culture cassettes (Mabio, Tourcoing, France) in normoxia (21% O2) or hypoxia chambers (1% O2; Sanyo, Avon, France).
SEMA3C stimulation experiments
Cultured pre-adipocytes and adipocytes were stimulated, during the first 6 or last 2 days of differentiation, respectively, with 1–500 ng/ml recombinant human SEMA3C-Fc-fusion protein (5570-S3-050; R&D Systems). For pre-adipocytes, medium containing recombinant SEMA3C was changed on the third day of incubation. Following stimulation, conditioned culture medium was collected and cells were lysed for total RNA or effects on glucose transport [29] and lipolysis [30] were evaluated exactly as described. Experiments were performed in duplicate or triplicate with untreated cells as control. Incubations with an Fc:fusion-control protein (ALX-203-004-C050; Enzo Life Sciences, Farmingdale, NY, USA) were used as a negative control to rule out possible Fc-mediated effects on gene expression. No alterations in mRNA levels for SEMA3C-regulated genes were observed (ESM Fig. 4).
Statistical analysis
Data shown in the figures are mean±SEM. For tables, results are presented as mean±SD, range, and/or fold change as detailed. For datasets that were not normally distributed, log10 transformed values were used. Results were analysed with appropriate parametric/non-parametric statistical tests, including Student’s paired/unpaired t test, Wilcoxon signed-rank test, simple/multiple regression analysis and analysis of variance using standard software packages.
Results
Identification of novel candidate adipokines regulated by weight alterations
Our analysis of human scWAT transcriptional profiles included three cohorts with either excess (cohort 1, lean vs obese) or reduced fat mass due to voluntary (cohort 2, before and after energy restriction) or involuntary (cohort 3, cancer with or without cachexia) weight loss. The expression of a large number of genes was altered by either obesity or weight loss (data not shown). However, only 112 genes were regulated, predominantly in a reciprocal manner, in all three cohorts (ESM Table 3). Thus, the genes that were upregulated in obesity were mostly downregulated after weight loss and vice versa. GO analysis of these genes demonstrated that the pathways involved in ECM formation and development/organ morphogenesis were particularly enriched (both upregulated in obesity; results not shown). To determine which of these genes encoded proteins secreted by adipocytes, we cross-matched the list of weight-regulated genes with data from the combined transcriptome and secretome analyses of cell fractions present in human WAT (cohorts 4 and 5, respectively). Out of the 112 candidates, only six (SEMA3C, NQO1, ABHD5, UCHL1, FMOD and GLUL) were found to be selectively transcribed and secreted by adipocytes and to contain a predicted signal peptide sequence (ESM Table 3). Five of these six genes encoded proteins that had been identified in recently published human adipocyte secretome screens (UCHL1, FMOD and GLUL) and/or functionally studied in human adipocytes/WAT (NQO1 and ABHD5). A single gene, SEMA3C, remained as a potentially novel adipokine not previously characterised in human WAT. Further studies were therefore focused on SEMA3C.
Mapping of class 3 semaphorin expression in human adipose tissue
Since little is known about the class 3 SEMA family in human WAT, we mapped the expression of SEMA3s in cohorts 1–3. Although signals for all seven ligands were detected and two of the family members were regulated by either obesity (SEMA3G) or loss of fat mass (SEMA3B), only SEMA3C was regulated in all three cohorts (ESM Table 4). The results in obesity and weight loss were confirmed by quantitative RT-PCR, demonstrating that scWAT SEMA3C mRNA levels were increased in obesity and the metabolic syndrome (cohort 6, Fig. 1a) and were reduced upon voluntary weight loss (by bariatric surgery, cohort 7, Fig. 1b) and involuntary weight loss (cachexia, cohort 3, Fig. 1c).
Human adipose SEMA3C mRNA expression. Expression of SEMA3C mRNA in subcutaneous WAT was compared between lean (LE) and obese (OB) participants and participants with the metabolic syndrome (MS) (cohort 6, n = 24) (a), before and after bariatric surgery (cohort 7, n = 13) (b) and between cancer patients with (CC) or without (weight stable [WS]) cancer cachexia (cohort 3, n = 27) (c). SEMA3C mRNA expression was assessed during adipogenic differentiation of human pre-adipocytes in vitro (d), in intact subcutaneous adipose tissue (AT) and paired samples of isolated, mature adipocytes (Adipo) (e) and in adipocytes and corresponding SVF (f). Results are presented as fold change±SEM relative to control participants. Data are means±SEM. *p < 0.05, **p < 0.01, ***p < 0.001
SEMA3C is a novel adipokine predominantly expressed in fat cells
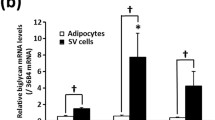
Adipose tissue is composed of several cell types including adipocytes, leucocytes and endothelial and progenitor cells. We therefore performed an extensive mapping of SEMA3C expression and secretion in human scWAT and distinct cell populations. SEMA3C mRNA expression increased during adipocyte differentiation in vitro (Fig. 1d) and was higher in isolated adipocytes than in paired samples of either intact WAT (cohort 8, Fig. 1e) or SVF (cohort 4, Fig. 1f). The presence of SEMA3C protein in WAT was further established by immunohistochemistry which detected the protein in both adipocytes and the stroma (ESM Fig. 3). Moreover, the secretion of SEMA3C from WAT explants was time dependent (cohort 9, Fig. 2a) and was significantly higher in samples from obese vs non-obese individuals (cohort 10, Fig. 2b). Since several SEMA3s, including SEMA3C, have been shown to be regulated by hypoxia in prostate cancer cells [31], we assessed whether SEMA3C secretion could be influenced by oxygen tension. However, following a 24 h incubation in either hypoxic (1%) or normoxic (21%) conditions, no differences in SEMA3C protein levels were observed in conditioned media from human adipocytes (cohort 11, data not shown). Additionally, in a recently published microarray study performed in human adipocytes [32], incubation under hypoxic conditions had no effect on SEMA3C expression, although SEMA3A expression was inhibited (2.9-fold reduction in mRNA level over 24 h).
Human adipose SEMA3C secretion. SEMA3C protein secretion was determined by ELISA in intact adipose tissue incubates (n = 6) at 1, 2 and 3 h (a) and intact tissue incubates from obese (OB) and non-obese (nOB) participants (b). All values were within the range of the standard curve. In part (b), values were corrected for fat-cell number and incubation time (to allow quantitative comparisons between groups). (c) The secreted levels of SEMA3C in part (b) were correlated to HOMAIR by multiple regression analysis using BMI as covariate (p value, 0.0017; r value, 0.441). The graph depicts a simple regression. Data are means±SEM. *p < 0.05, ***p < 0.001
Adipose tissue SEMA3C levels are associated with insulin resistance and fat-cell morphology
While the results obtained so far demonstrate that SEMA3C is an adipokine that is regulated by weight changes, they do not yield any information on the role of SEMA3C in WAT function. We assessed whether SEMA3C mRNA levels were associated with any clinical variables in cohort 1. SEMA3C correlated positively with circulating glucose and insulin levels, measurements of whole-body insulin resistance (HOMA of insulin resistance [HOMAIR] [33] and the rate constant for the insulin tolerance test [KITT]) and fat-cell size and morphology independent of BMI (Table 1). In addition, the levels of SEMA3C secreted from WAT explants correlated significantly with HOMAIR independent of BMI (Fig. 2c). These findings encouraged us to evaluate the function of SEMA3C in vitro.
Functional characterisation of SEMA3C in primary human adipocytes
The effect of recombinant SEMA3C protein on primary cultures of human adipocytes was determined using concentrations in the range of 10–500 ng/ml. These concentrations were chosen based on ranges used in published studies in non-adipose cells [34]. Adipocytes were incubated with SEMA3C for 48 h and the effect on glucose transport, lipolysis and the expression of genes regulating lipid oxidation was determined. However, no significant effect on any of these variables was observed (ESM Fig. 5). The lack of response prompted us to determine whether SEMA3C receptors are expressed in differentiated human adipocytes. As detailed in Fig. 3a, the expression of all receptors, except for PLXNA2 (which was found at very low levels), was markedly downregulated during adipocyte differentiation, suggesting that SEMA3C may act on adipocyte progenitor cells (pre-adipocytes) present within WAT.
SEMA3C receptor levels and effects of SEMA3C in primary human pre-adipocytes. (a) mRNA expression of the SEMA3C receptor PLXNs (PLXNA1-2, -4 and -D1) and co-receptors NRPs (NRP1-2) during adipogenesis (n = 12). All receptors/co-receptors, except for PLXNA2, were significantly downregulated during differentiation († p < 0.0001for all, using repeated measures ANOVA). According to microarray results (data not shown), PLXNA3 expression was extremely low in human adipose tissue and therefore not studied. Black circles, NRP1; black squares, NRP2; black triangles, PLXNA1; white circles, PLXNA2; white squares PLXNA4 and white triangles, PLXND1. Data are means±SEM. (b) Five of the genes were significantly altered at the mRNA level (Table 2) and were evaluated at the protein level by determining the secretion in differentiating human adipocytes incubated with SEMA3C (100 ng/ml) for 6 days. The release of all but one (fibronectin) was increased by SEMA3C. Data are means±SEM (n = 6); *p < 0.05 compared with control (Ctrl). (c) The effects of SEMA3C in promoting functional ECM matrix were determined by immunofluorescence in human pre-adipocytes after 6 days of incubation with SEMA3C (100 ng/ml). Cells incubated with SEMA3C displayed a significantly more pronounced staining for both fibronectin (FN1) and collagen compared with control cells
SEMA3C regulates the production of ECM-related components in primary human pre-adipocytes
Pre-adipocytes are not only essential for adipogenesis. Recent studies demonstrate that human pre-adipocytes cultured in an inflammatory context (such as conditioned media from WAT macrophages) produce several factors that could promote the generation of WAT fibrosis [26]. Several structural components (e.g. collagen I/VI [encoded by COL1A1/COL6A1], fibronectin 1 [FN1] and elastin [ELN]), matricellular proteins (e.g. CTGF [encoded by CTGF] and TGF-β1 [TGFB1]) and inflammatory cyto-/chemokines (e.g. IL6 [encoded by IL6]) have been implicated in tissue remodelling and the development of fibrosis. We determined whether recombinant SEMA3C affected gene/protein expression of factors involved in differentiation, inflammation and ECM composition in human primary pre-adipocytes. Different concentrations of SEMA3C had no effect on adipogenesis determined by either lipid accumulation (assessed by microscopy, data not shown) or gene expression (of FABP4, CEBPA and PPARG, results not shown). Moreover, the expression of pro-inflammatory factors, including TNF and CCL2, -3 or -5, was unaltered (Table 2). In contrast, SEMA3C increased mRNA levels of genes known to be associated with ECM remodelling and/or fibrosis (Table 2), including structural components (COL1A1, ELN and FN1), matricellular factors and cytokines (CTGF, TGFB1 and IL6). These effects were confirmed at the protein level by ELISA (Fig. 3b), whereby levels of CTGF were found to be particularly induced (about fivefold). Moreover, immunofluorescence assays showed a more distinct extracellular network of fibronectin and collagen I induced by the addition of SEMA3C (Fig. 3c). The clinical relevance of these results was supported by the fact that SEMA3C mRNA levels correlated positively with interstitial fibrosis as evaluated by immunohistochemistry, at least in subcutaneous WAT of severely obese participants (r = 0.546, p = 0.0104, cohort 12).
Discussion
By combining human adipose transcriptome and secretome data, we identified six potentialadipokines that were altered by changes in WAT mass. Out of these, five were described in previous secretome analyses and/or functionally studied in human adipocytes. Only SEMA3C was novel (i.e. previously not characterised in human WAT/adipocytes). We found that SEMA3C was predominantly expressed in fat cells and that its expression and secretion correlated with hypertrophic WAT (fewer but larger fat cells, a phenotype associated with in vivo insulin resistance [18, 35]) and independent measures of in vivo whole-body insulin resistance (HOMAIR, KITT), regardless of BMI. Recombinant SEMA3C did not have any direct metabolic effects on human adipocytes in culture. This could be due either to the overall reduction in cognate receptor gene expression during differentiation or to a lack of effect on pathways controlling glucose/lipid handling. In contrast, in human pre-adipocytes, SEMA3C stimulated the mRNA/protein expression of structural and matricellular genes, resulting in ECM remodelling. Pre-adipocytes have recently been demonstrated to represent major cellular mediators promoting WAT fibrosis deposition in the pro-inflammatory micro-environment of obesity [26]. We observed that SEMA3C stimulates the production of several remodelling factors implicated in fibrosis deposition, including IL6 and TGF-β1 and CTGF, the latter two constituting important growth factors shown to be dysregulated in diseases with major alterations in tissue remodelling (such as systemic sclerosis) [36]. In the context of obesity, the finding that SEMA3C expression correlated with human WAT fibrosis, at least in adipose samples from severely obese individuals, lends pathophysiological relevance to our data. Taken together, SEMA3C may influence insulin sensitivity via modified ECM deposition/interstitial fibrosis, a hypothesis which needs further experimental validation, preferably in an in vivo model.
Although human WAT displays a high adipocyte turnover, the total number of fat cells in adults remains constant over time and is unaltered by voluntary/involuntary weight reduction [37, 38]. The production of ECM from fibroblast-like adipose precursor cells is therefore essential in order to allow dynamic changes in fat-cell volume while retaining tissue stability. The relationship between fat-cell size and ECM remodelling was recently shown in WAT from healthy growing children [39]. However, ECM overproduction in obesity may have a negative impact on WAT plasticity and function through the development of interstitial fibrosis. The latter has been proposed to reduce oxygenation, leading to local hypoxia, which in turn attenuates adipogenesis and induces insulin resistance [40]. Hypoxia per se stimulates the production of ECM and this leads to a vicious circle further promoting a dysfunctional WAT [41]. These hypotheses are supported by findings in animal models demonstrating that knockout of ECM components (e.g. collagen type VI α1) protects against insulin resistance in diet-induced obesity [42] while overexpression of hypoxia-inducible factor-1α increases interstitial fibrosis and insulin resistance [41]. In line with these findings, fibrosis has been demonstrated in WAT of obese insulin-resistant individuals [43]. Altogether, this suggests that the amount and composition of the adipose interstitial compartment is important for tissue function/insulin sensitivity and that ECM production needs to be tightly regulated, most probably via dynamic crosstalks between adipocytes and adjacent cells. However, the signals that control ECM production in conditions of fat mass gain/loss have not been extensively studied [1, 40]. The mechanisms that regulate SEMA3C expression in human adipocytes are unclear. It is unlikely that increases in circulating glucose or NEFA levels stimulate SEMA3C expression in human WAT. This notion is supported by the finding that SEMA3C mRNA was reduced by weight loss following both bariatric surgery and cancer cachexia, the latter a condition characterised by increased NEFA and unaltered glucose levels [13]. Since SEMA3s, including SEMA3C, have been shown to be regulated by hypoxia in prostate cancer cells [31], it is tempting to speculate that hypoxia may induce ECM production via SEMA3C. In our experimental set-up, neither the expression nor secretion of SEMA3C by cultured human adipocytes was affected by reduced oxygen tension, suggesting that SEMA3C production by fat cells is regulated via hypoxia-independent mechanisms which need to be further investigated. The observation that SEMA3C expression correlated significantly with adipocyte morphology (a measure that is independent of body fat mass as discussed [18]) demonstrates that there is a link between relative fat-cell size and SEMA3C levels. Fat-cell morphology is determined by adipocyte [18] and lipid turnover [44]. However, the transcriptional networks that regulate these processes have not yet been determined and their potential role in regulating SEMA3C requires further investigation.
There are relatively few published studies on SEMA3C in general (<50 publications indexed in PubMed to date) and it has been studied predominantly in non-adipose tissue. Class 3 semaphorins have primarily been implicated in axon guidance and SEMA3C has been shown to act both as a neurochemoattractant and -repellent in vitro [45]. In addition, SEMA3C appears to play a role in angiogenesis, since it stimulates the proliferation of murine glomerular endothelial cells [34]. The physiological relevance of these findings is corroborated by the phenotype of SEMA3C −/− mice, which die shortly after birth due to congenital cardiovascular malformations [46]. We incubated human adipose-derived endothelial cells (CD34+/CD31+) with SEMA3C in vitro, but were unable to observe any pro-proliferative effects (not shown). However, this does not exclude the possibility that SEMA3C may have an impact on other aspects of angiogenesis/endothelial cell function in human WAT.
While SEMA3C displayed marked effects in vitro, the signalling pathways remain to be elucidated. Results in recent years suggest that SEMAs, including SEMA3s, signal via multimeric receptor complexes which activate intracellular signalling pathways that have only been partially unravelled [11]. It is at present unclear which receptor(s) mainly confer the effects of SEMA3C in human adipocytes; however, a comparison of relative mRNA levels shows that NRP1 and -2 and PLXND1 are the most highly expressed isoforms in WAT (results not shown). A mapping in stroma-vascular-derived cells from human WAT (adipocyte progenitor cells, macrophages, lymphocytes and endothelial cells) demonstrated a similar expression pattern with clearly detectable levels of NRP1 and -2 and PLXND1 mRNA in all fractions, while PLXNA1 and -A4 were expressed at considerably lower levels and the latter primarily in progenitor and endothelial cells (ESM Fig. 6). PLXNA2 expression was below the detection limit (Ct values >32, data not shown). This suggests that SEMA3C may also have effects in other cell types present in human WAT. However, future studies are needed to define the ligand–receptor stoichiometry, signalling pathways and SEMA3C-mediated effects in different cells of human WAT.
A caveat in our study is that the majority of the participants were women. However, in the men that were enrolled, there was no indication that sex influenced SEMA3C expression. Taken together, our data suggest that SEMA3C is a novel adipokine, the expression of which correlates significantly with weight change, fat-cell hypertrophy, adipose fibrosis and insulin resistance in humans. While recombinant SEMA3C does not affect insulin sensitivity in adipocytes directly, it stimulates the production and release of structural and matricellular proteins in pre-adipocytes. We hypothesise that SEMA3C may be a factor that enables WAT remodelling, disturbances of which may contribute to the development of insulin resistance and type 2 diabetes.
Abbreviations
- CTGF:
-
Connective tissue growth factor
- ECM:
-
Extracellular matrix
- ELN:
-
Elastin
- GO:
-
Gene ontology
- HOMAIR :
-
HOMA of insulin resistance
- KITT :
-
Rate constant for the insulin tolerance test
- NRP:
-
Neuropilin
- PLXN:
-
Plexin
- SAM:
-
Significance analysis of microarrays
- scWAT:
-
Subcutaneous WAT
- SEMA3C:
-
Semaphorin 3C
- SVF:
-
Stroma-vascular fraction
- TGF-β1:
-
Transforming growth factor-β1
- WAT:
-
White adipose tissue
References
Sun K, Kusminski CM, Scherer PE (2011) Adipose tissue remodeling and obesity. J Clin Invest 121:2094–2101
Lofgren P, Andersson I, Adolfsson B et al (2005) Long-term prospective and controlled studies demonstrate adipose tissue hypercellularity and relative leptin deficiency in the postobese state. J Clin Endocrinol Metab 90:6207–6213
Lofgren P, Hoffstedt J, Naslund E, Wiren M, Arner P (2005) Prospective and controlled studies of the actions of insulin and catecholamine in fat cells of obese women following weight reduction. Diabetologia 48:2334–2342
Trayhurn P, Drevon CA, Eckel J (2011) Secreted proteins from adipose tissue and skeletal muscle—adipokines, myokines and adipose/muscle cross-talk. Arch Physiol Biochem 117:47–56
Kim J, Choi YS, Lim S et al (2010) Comparative analysis of the secretory proteome of human adipose stromal vascular fraction cells during adipogenesis. Proteomics 10:394–405
Lehr S, Hartwig S, Lamers D et al (2012) Identification and validation of novel adipokines released from primary human adipocytes. Mol Cell Proteomics 11(M111):010504
Rosenow A, Arrey TN, Bouwman FG et al (2010) Identification of novel human adipocyte secreted proteins by using SGBS cells. J Proteome Res 9:5389–5401
Zhong J, Krawczyk SA, Chaerkady R et al (2010) Temporal profiling of the secretome during adipogenesis in humans. J Proteome Res 9:5228–5238
Zvonic S, Lefevre M, Kilroy G et al (2007) Secretome of primary cultures of human adipose-derived stem cells: modulation of serpins by adipogenesis. Mol Cell Proteomics 6:18–28
Giordano A, Cesari P, Capparuccia L, Castellucci M, Cinti S (2003) Sema3A and neuropilin-1 expression and distribution in rat white adipose tissue. J Neurocytol 32:345–352
Sharma A, Verhaagen J, Harvey AR (2012) Receptor complexes for each of the class 3 semaphorins. Front Cell Neurosci 6:28
Mejhert N, Galitzky J, Pettersson AT et al (2010) Mapping of the fibroblast growth factors in human white adipose tissue. J Clin Endocrinol Metab 95:2451–2457
Agustsson T, Ryden M, Hoffstedt J et al (2007) Mechanism of increased lipolysis in cancer cachexia. Cancer Res 67:5531–5537
Alberti KG, Eckel RH, Grundy SM et al (2009) Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation 120:1640–1645
Alberti KG, Zimmet P, Shaw J (2005) The metabolic syndrome—a new worldwide definition. Lancet 366:1059–1062
Curat CA, Miranville A, Sengenes C et al (2004) From blood monocytes to adipose tissue-resident macrophages: induction of diapedesis by human mature adipocytes. Diabetes 53:1285–1292
Miranville A, Heeschen C, Sengenes C, Curat CA, Busse R, Bouloumie A (2004) Improvement of postnatal neovascularization by human adipose tissue-derived stem cells. Circulation 110:349–355
Arner E, Westermark PO, Spalding KL et al (2010) Adipocyte turnover: relevance to human adipose tissue morphology. Diabetes 59:105–109
Duffaut C, Zakaroff-Girard A, Bourlier V et al (2009) Interplay between human adipocytes and T lymphocytes in obesity: CCL20 as an adipochemokine and T lymphocytes as lipogenic modulators. Arterioscler Thromb Vasc Biol 29:1608–1614
Pettersson AT, Laurencikiene J, Mejhert N et al (2010) A possible inflammatory role of twist1 in human white adipocytes. Diabetes 59:564–571
Decaunes P, Esteve D, Zakaroff-Girard A, Sengenes C, Galitzky J, Bouloumie A (2011) Adipose-derived stromal cells: cytokine expression and immune cell contaminants. Methods Mol Biol 702:151–161
Tusher VG, Tibshirani R, Chu G (2001) Significance analysis of microarrays applied to the ionizing radiation response. Proc Natl Acad Sci USA 98:5116–5121
Zhang B, Kirov S, Snoddy J (2005) WebGestalt: an integrated system for exploring gene sets in various biological contexts. Nucleic Acids Res 33:W741–W748
Klimcakova E, Roussel B, Kovacova Z et al (2011) Macrophage gene expression is related to obesity and the metabolic syndrome in human subcutaneous fat as well as in visceral fat. Diabetologia 54:876–887
Dahlman I, Kaaman M, Olsson T et al (2005) A unique role of monocyte chemoattractant protein 1 among chemokines in adipose tissue of obese subjects. J Clin Endocrinol Metab 90:5834–5840
Keophiphath M, Achard V, Henegar C, Rouault C, Clement K, Lacasa D (2009) Macrophage-secreted factors promote a profibrotic phenotype in human preadipocytes. Mol Endocrinol 23:11–24
Divoux A, Tordjman J, Lacasa D et al (2010) Fibrosis in human adipose tissue: composition, distribution, and link with lipid metabolism and fat mass loss. Diabetes 59:2817–2825
Henegar C, Tordjman J, Achard V et al (2008) Adipose tissue transcriptomic signature highlights the pathological relevance of extracellular matrix in human obesity. Genome Biol 9:R14
Mejhert N, Laurencikiene J, Pettersson AT et al (2010) Role of receptor-interacting protein 140 in human fat cells. BMC Endocr Disord 10:1
Stenson BM, Ryden M, Venteclef N et al (2011) Liver X receptor (LXR) regulates human adipocyte lipolysis. J Biol Chem 286:370–379
Blanc V, Nariculam J, Munson P et al (2011) A role for class 3 semaphorins in prostate cancer. Prostate 71:649–658
Mazzatti D, Lim FL, O'Hara A, Wood IS, Trayhurn P (2012) A microarray analysis of the hypoxia-induced modulation of gene expression in human adipocytes. Arch Physiol Biochem 118:112–120
Wahrenberg H, Hertel K, Leijonhufvud BM, Persson LG, Toft E, Arner P (2005) Use of waist circumference to predict insulin resistance: retrospective study. Bmj 330:1363–1364
Banu N, Teichman J, Dunlap-Brown M, Villegas G, Tufro A (2006) Semaphorin 3C regulates endothelial cell function by increasing integrin activity. Faseb J 20:2150–2152
Hoffstedt J, Arner E, Wahrenberg H et al (2010) Regional impact of adipose tissue morphology on the metabolic profile in morbid obesity. Diabetologia 53:2496–2503
Abraham D (2008) Connective tissue growth factor: growth factor, matricellular organizer, fibrotic biomarker or molecular target for anti-fibrotic therapy in SSc? Rheumatology (Oxford) 47(Suppl 5):v8–9
Ryden M, Agustsson T, Laurencikiene J et al (2008) Lipolysis—not inflammation, cell death, or lipogenesis—is involved in adipose tissue loss in cancer cachexia. Cancer 113:1695–1704
Spalding KL, Arner E, Westermark PO et al (2008) Dynamics of fat cell turnover in humans. Nature 453:783–787
Tam CS, Tordjman J, Divoux A, Baur LA, Clement K (2012) Adipose tissue remodeling in children: the link between collagen deposition and age-related adipocyte growth. J Clin Endocrinol Metab 97:1320–1327
Divoux A, Clement K (2011) Architecture and the extracellular matrix: the still unappreciated components of the adipose tissue. Obes Rev 12:e494–503
Halberg N, Khan T, Trujillo ME et al (2009) Hypoxia-inducible factor 1alpha induces fibrosis and insulin resistance in white adipose tissue. Mol Cell Biol 29:4467–4483
Khan T, Muise ES, Iyengar P et al (2009) Metabolic dysregulation and adipose tissue fibrosis: role of collagen VI. Mol Cell Biol 29:1575–1591
Spencer M, Yao-Borengasser A, Unal R et al (2010) Adipose tissue macrophages in insulin-resistant subjects are associated with collagen VI and fibrosis and demonstrate alternative activation. Am J Physiol Endocrinol Metab 299:E1016–1027
Arner P, Bernard S, Salehpour M et al (2011) Dynamics of human adipose lipid turnover in health and metabolic disease. Nature 478:110–113
Derijck AA, Van Erp S, Pasterkamp RJ (2010) Semaphorin signaling: molecular switches at the midline. Trends Cell Biol 20:568–576
Feiner L, Webber AL, Brown CB et al (2001) Targeted disruption of semaphorin 3C leads to persistent truncus arteriosus and aortic arch interruption. Development 128:3061–3070
Acknowledgements
We thank T. C. Walther (Department of Cell Biology, Yale University School of Medicine, New Haven, CT, USA) and P. Arner (Department of Medicine [H7], Karolinska Institutet, Stockholm, Sweden) for important input and support as well as E. Dungner, E. Sjölin, K. Wåhlén, G. Åström, B.-M. Leijonhufvud, K. Hertel and Y. Widlund (Department of Medicine [H7], Karolinska Institutet) for excellent technical assistance.
Funding
This work was supported by several grants: to M. Rydén from the Swedish Diabetes Association, the Novo Nordisk Foundation, the Swedish Cancer Foundation, DiabetesWellness, the Swedish Medical Association, the Swedish Research Council, ADAPT (HEALTH-F2-2008-201100), NordForsk (SYSDIET-070014) and the Strategic Research Programme in Diabetes at the Karolinska Institutet; to F. Wilfling from the Boehringer Ingelheim Fonds and to K. Clément and D. Langin from Fondation pour la Recherche Médicale and the French National Agency of Research (ANR Adipofib and LipOb).
Duality of interest
The authors declare that there is no duality of interest associated with this manuscript.
Contribution statement
NM and MR designed the study, performed the in vitro experiments, analysed the data and wrote the manuscript. NM, ID and FW identified genes regulated by weight changes. C-IK, NV, VS and D. Langin collected adipose tissue and measured SEMA3C mRNA levels in cohorts 4 and 6. C-IK, NV, D. Langin and AB collected adipose tissue and performed secretome analysis in cohort 5. NM and MR measured secreted SEMA3C protein levels in cohorts 9 and 10. MR and EN collected adipose tissue and assessed SEMA3C mRNA levels in cohorts 1, 3, 7 and 8. PT, DE, JG and AB measured SEMA3C levels in cohort 11. KC and JT collected cohort 12, JT quantified fibrosis and VP and D. Lacasa performed the mRNA measurements of SEMA3C in the same cohort. VP, D. Lacasa and PL performed the immunocyto-/histochemistry experiments. All authors contributed to data interpretation, reviewed the manuscript and approved the final version.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM Table 1
(PDF 7.49 kb)
ESM Table 2
(PDF 6.96 kb)
ESM Table 3
(PDF 49.6 kb)
ESM Table 4
(PDF 7.04 kb)
ESM Fig. 1
Evaluation of ELISA specificity. Human in vitro differentiated adipocytes isolated from six independent donors were transfected with SEMA3C-targeting siRNA oligonucleotides (siSEMA3C) or non-silencing control siRNA (N.C.). After 48 h, SEMA3C a) mRNA levels were determined by qPCR and b–e) secreted protein levels in conditioned media were assessed by Western blot and ELISA. For Western blots, conditioned media was concentrated and ∼15 μg of total secreted protein was loaded. In cells from donors 1 and 2, SEMA3C was detected using two different α-SEMA3C antibodies (in panel b: N-20 and panel c: MAB1728, see ESM for details). Both antibodies detected two bands out of which the lower (indicated by the arrow) corresponded to the predicted size of SEMA3C protein. d) Quantification and statistical evaluation of band intensities (O.D) in panel c showed a statistically significant (p < 0.05) ∼ 50% reduction in band intensities e) ELISA of conditioned media showed that SEMA3C levels in the N.C. cells were within the range of the standard curve (i.e. >78 pg/ml, represented by the upper dashed line), while the levels in the siSEMA3C samples were significantly reduced (p < 0.05) by ∼50 %, albeit above the lowest detectable level (>27.7 pg/ml, represented by the lower dashed line). f) To control for possible differences in loading and/or non-specific RNAi effects on protein secretion, the secreted levels of adiponectin were determined by ELISA in the same samples as in panel e. There was no statistically significant difference comparing the two treatments. ***p < 0.001; *p < 0.05. (PDF 57.6 kb)
ESM Fig. 2
Immunohistochemical analysis of adipose tissue fibrosis. Representative microphotographs showing scWAT stained with picrosirius red. (PDF 457 kb)
ESM Fig. 3
SEMA3C immunohistochemistry. a) SEMA3C protein was detected in adipocytes and the extracellular matrix using a α-SEMA3C antibody (N-20, Santa Cruz Biotechnology. Similar stainings were obtained using another α-SEMA3C antibody (panel not shown). b) Control experiments were performed by replacing the α-SEMA3C antibody with pre-immune sera. (PDF 100 kb)
ESM Fig. 4
FC:fusion control protein stimulation. Lack of effects of recombinant Fc:fusion control protein on gene expression. Keys are as follows: blue circles, COL1A1; red circles, CTGF; green circles, ELN; purple circles, FN1; yellow circles, IL6 and turquoise circles, TGFB1. (PDF 17 kb)
ESM Fig. 5
Effects of SEMA3C in primary human adipocytes. Lack of effects of recombinant SEMA3C protein (10-500 ng/ml) on a) basal (white bars) and insulin-stimulated (black bars) glucose transport, b) glycerol release and c) genes regulating lipid oxidation (black circles, ACACB acetyl-CoA carboxylase beta; white circles, CIDEA cell death-inducing DFFA-like effector a; black squares, CPT1B - carnitine palmitoyltransferase 1B and white squares, ACADM acyl-CoA dehydrogenase, C-4 to C-12 straight chain). Results are presented as fold change±SEM relative to control (ctrl). (PDF 16.3 kb)
ESM Fig. 6
SEMA3 receptor mRNA levels in adipose stroma-vascular fractions. Neuropilin (NRP) and plexin (PLXN) mRNA levels were measured in adipose stroma-vascular fractions including lymphocytes (black bars), macrophages (grey bars), endothelial (white bars) and progenitor cells (striped bars). (PDF 10.8 kb)
ESM Methods
(PDF 67.5 kb)
Rights and permissions
About this article
Cite this article
Mejhert, N., Wilfling, F., Esteve, D. et al. Semaphorin 3C is a novel adipokine linked to extracellular matrix composition. Diabetologia 56, 1792–1801 (2013). https://doi.org/10.1007/s00125-013-2931-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00125-013-2931-z