Abstract
Aims/hypothesis
Type 2 diabetes has been associated with an increased risk of cancer. This study examines the effect of more vs less intensive glucose control on the risk of cancer in patients with type 2 diabetes.
Methods
All 11,140 participants from the Action in Diabetes and Vascular Disease: Preterax and Diamicron-MR Controlled Evaluation (ADVANCE) trial (ClinicalTrials.gov NCT00145925) were studied. Cancer incidence and cancer mortality was compared in groups randomised to intensive or standard glucose control. Information on events during follow-up was obtained from serious adverse event reports and death certificates. HRs (95% CI) were calculated for all cancers, all solid cancers, cancer deaths and site-specific cancers.
Results
After a median follow-up of 5 years, 363 and 337 cancer events were reported in the intensive and standard control groups, respectively (incidence 1.39/100 person-years [PY] and 1.28/100 PY; HR 1.08 [95% CI 0.93–1.26]). The incidences of all solid cancers and cancer deaths were 1.25/100 PY and 0.15/100 PY in the intensive group and 1.15/100 PY and 0.13/100 PY in the standard group (HR 1.09 [95% CI 0.93–1.27] for solid cancers, and 1.17 [0.75–1.84] for cancer death). Across all the major organ systems studied, no significant differences in the cancer incidences were observed in the intensive and standard control groups.
Conclusions/interpretations
More intensive glucose control achieved with a regimen that included greater use of gliclazide, insulin, metformin and other agents, did not affect the risk of cancer events or death in patients with type 2 diabetes.
Similar content being viewed by others
Introduction
There have been many reports of increased risk of cancer in patients with diabetes including cancers of the pancreas, liver, breast, colorectum, urinary tract and female reproductive organs [1–3]. Cancer mortality has also been reported to be increased [1, 4]. In type 2 diabetes, these increases in cancer risk have been associated with inflammation, obesity, insulin resistance, physical inactivity, elevated glucose levels and the administration of exogenous insulin [1, 5–9]. Yang and colleagues reported a linear relationship between increasing HbA1c levels and cancer risk above HbA1c levels of 6.5% [7]. It has been proposed that reducing glucose levels may reduce cancer risk [5–7].
To date, observational studies have examined the effects of intensive glucose control, metformin therapy, insulin therapy and cancer outcomes with inconsistent findings [7, 10–14]. Some have observed decreased risks of cancer with insulin therapy, whereas others have reported increased risks of cancer associated with insulin therapy [7, 10–15]. Discrepancies have been linked to differences in study design, to the presence of residual confounding and to differences in the likely mechanisms by which various therapies lower glucose levels [7, 16, 17]. It also remains unclear whether the effects observed are attributable to lower glucose levels or to the effects of the medications used to achieve greater glucose lowering [7, 16, 17]. Indeed, observational studies cannot reliably determine whether more intensive glucose control, or the treatments used to achieve more intensive glucose lowering affect the risk of cancer. Because changes to therapy might well be intimately related to the patient's health, observational studies linking cancer risk to therapy over time are likely to suffer from allocation bias [18, 19]. More robust evidence might be derived from randomised controlled trials.
The objective of this study was to determine whether the intensity of glucose control influences the risk of cancer in patients with type 2 diabetes. To this end, data from the Action in Diabetes and Vascular disease: Preterax and Diamicron MR Controlled Evaluation (ADVANCE) trial, a randomised controlled trial, was analysed to examine cancer risk in patients with type 2 diabetes randomised to intensive glucose control compared with standard glucose control over a 5-year period [20, 21].
Methods
Study design
ADVANCE was a factorial trial of blood pressure lowering and intensive glucose control conducted in 215 centres in 20 countries between 2001 and 2008 [20–22]. Participants had been diagnosed with type 2 diabetes after age 30 years and were aged at least 55 years at study enrolment. In the glucose control group, 5,571 participants were randomised to intensive glucose control and 5,569 participants were randomised to standard guideline-based glucose control [21]. At study entry, people suffering from life-threatening non-vascular disease other than diabetes and its complications, including cancer, were deemed ineligible. Participant characteristics have been described in detail elsewhere [20, 21]. Those assigned to intensive glucose control received gliclazide modified release (MR) (30–120 mg/day) and other drugs, as required, to achieve an HbA1c level of less than or equal to 6.5% [21]. Those assigned to standard glucose control received treatments based on local guidelines, with the exception of gliclazide MR, which was substituted with any other sulfonylurea [20–22]. Approval for the trial was obtained from the institutional ethics committee of each participating centre and all participants provided written informed consent.
Outcome measures
All serious adverse events, as well as death from any cause, were recorded during follow-up. Cancer outcomes were pre-specified as events of interest, and coded according to the International Classification of Diseases, tenth edition (ICD-10): neoplasm C00-D48; malignant neoplasm C00-C97; malignant neoplasm except lymphoid tissue C00-C75; lip, oral cavity and pharynx C00-C14; digestive organs C15-C26; respiratory organs C30-C39; breast C50; female genital organs C51-C58; male genital organs C60-C63; cancer from lymphoid tissue C81-C96. The date of onset of the event was also recorded. The cause of each death was adjudicated by an independent endpoint committee blinded to treatment allocation.
Statistical analysis
Follow-up time for each participant was calculated as time from registration date to date of cancer event or censoring. The first cancer event was included in the analysis. The incidences of all malignant neoplasms and each specific cancer type were calculated by treatment assignment as the rate per 100 person-years (PY). HRs (95% CI) for randomised treatment effects were estimated using unadjusted Cox proportional hazard models. Kaplan–Meier plots were constructed to demonstrate time-dependent cancer-free survival. Subgroup analyses examined the effects of HbA1c level at study entry. All analyses were performed using SAS version 9.1 (SAS Institute).
Results
The characteristics of participants assigned to gliclazide MR-based intensive glucose control and to guideline-based standard glucose control were similar at baseline (Table 1). At the end of follow-up, HbA1c levels were lower in the intensive than the standard glucose control group, as expected, as a result of greater use of oral glucose lowering therapy and insulin (Table 1).
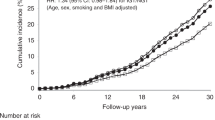
The median duration of follow-up was 5.0 years. Malignant neoplasms occurred in 363 participants assigned intensive glucose control and 337 participants assigned standard glucose control. This corresponds to 1.39 and 1.28 malignancies per 100 PY, respectively. The HR for intensive vs standard glucose control was 1.08 (95% CI 0.93–1.26) (Table 2). Malignant neoplasms classified as solid cancers occurred in 328 participants assigned intensive glucose control and 303 participants assigned standard glucose control. This corresponds to 1.25 and 1.15 solid cancers per 100 PY (HR 1.09 [95% CI 0.93–1.27]) (Table 2). The Kaplan–Meier cumulative event-free survival curve for all solid malignancies is shown in Figure 1. The incidence of all malignant neoplasms classified by major organ systems and by treatment assignment is shown in Table 2. Intensive glucose control was not associated with either an increased or a decreased risk of any specific type of major organ system cancer. The effects of treatment assignment did not significantly differ by entry HbA1c level (HbA1c ≤ 7.2%, HR 1.15 [95% CI 0.94–1.41]; HbA1c > 7.2%, HR 1.01 [95% CI 0.81–1.25], p for heterogeneity = 0.38).
Cancer deaths were reported for 41 participants assigned to intensive glucose control and 35 participants assigned to standard glucose control. This corresponds to a cancer mortality rate of 0.15 per 100 PY in the intensive control group as compared with 0.13 per 100 PY in the standard control group (HR 1.17, 95% CI 0.75–1.84) (Table 2). The Kaplan–Meier cumulative event-free survival curve for cancer mortality is shown in Figure 2.
Discussion
These analyses, which compared cancer events between groups randomised to intensive or standard glucose control, do not suggest that more intensive glucose control based on greater use of gliclazide, insulin, metformin and other agents is associated with an effect on cancer incidence or mortality. There were no significant differences in the risk of all malignant neoplasms, all solid malignant neoplasms, nor of any organ-specific cancers classified according to the ICD-10 codes for major organ systems. There was also no significant difference overall in cancer deaths between the groups assigned to more intensive vs less intensive glucose lowering.
The overall cancer event rate observed in our study of patients with long standing type 2 diabetes (1.33 per 100 PY) was similar to that of one large population-based study of people with diabetes (1.31 per 100 PY) [23] and slightly greater than that of another (1.21 per 100 PY) [24]. Notably, both these cohort studies also examined cancer incidence in individuals free of diabetes and reported lower overall event rates (1.19 and 0.77 per 100 PY, respectively).
Observational studies have produced complex and at times confusing findings regarding the risk of cancer in patients with diabetes [1–5]. Furthermore the mechanisms that might contribute to these effects are not clear. Some have suggested that the effects are mediated by increased insulin levels [25], whereas others have suggested that the effects are due to over-expression in cancer cells of the IGF-1 receptor, or the insulin receptor itself, especially the A isoform, which is uniquely able to bind both insulin and IGF-2 [2, 3, 26, 27]. In vitro studies have also suggested that hyperglycaemia per se may increase human breast cancer cell proliferation rates [28]. Because cancer cells are thought to be obligate glucose users, even modest elevations of glucose levels may drive cell metabolism and growth via the Warburg effect, which allows synthesis of amino acids, nucleotides and lipids for cell replication at the expense of ATP production [16].
The UK Prospective Diabetes Study (UKPDS), Action to Control Cardiovascular Risk in Diabetes (ACCORD) study and Veterans Affairs Diabetes Trial (VADT), with average follow-up of between 3.5 and 10 years, have individually reported no effects on the risk of cancer mortality, the most reliable single index of cancer incidence [29–32]. The more detailed randomised evidence from ADVANCE that we have presented here is consistent with the overall evidence from these trials. However, none of these trials were specifically designed as trials of cancer outcomes. Nevertheless, the pooled data from these trials of more vs less intensive glucose control (which achieved an HbA1c difference of 0.3–0.8% for trials reporting cancer incidence and 0.6–1.4% for trials reporting cancer mortality) do not support the premise that cancer incidence (pooled risk ratio [RR] 0.91 [95% CI 0.79–1.05]; I 2 = 0%) or mortality (pooled RR 1.00 [95% CI 0.81–1.24]; I 2 = 0%) is increased or decreased with more intensive glucose lowering [32].
Although the present results do not suggest any significant effects of intensive glucose lowering on cancer outcomes, there were some minor trends towards increases in cancer numbers across some organ systems in those assigned to intensive vs standard glucose control (Table 2). One possible explanation for this lies in the greater frequency of visits for those assigned intensive therapy (who were seen at least every 3 months, or even more often) compared with standard therapy (who were only seen every 6 months). Thus earlier diagnosis and detection of cancers may have occurred in those in the intensive control group as a result of the more frequent medical surveillance provided. Indeed in support of this, an increased rate of hospitalisation among those assigned intensive glucose control has previously been reported [21]. However, despite the possible greater ascertainment of some cancers in the intensive treatment group, the frequency of breast cancer was similar in both treatment groups. Another possible explanation for the trend toward increases in some cancers lies in the proportional differences in the use of glucose-lowering therapies in the two treatment groups, including use of insulin and metformin. Further analyses combining individual participant data from the major randomised trials of more intensive glucose lowering [21, 29–31] would provide greater power to address these questions.
The main strengths of this study include its examination of outcomes in groups randomly assigned to intensive or standard guidelines-based glucose control, its large patient population, spread across 20 countries worldwide, and detailed reporting of serious adverse events over a median of 5 years. This study has a number of limitations. It was not designed to specifically assess cancer outcomes so that cancer events were not routinely confirmed by pathology reports or validated against cancer registry data. The 5-year period of follow-up was too short for accurate determination of the risk of inducing new cancers. An effect of a larger difference in HbA1c between the treatment groups may also have been missed, although this would seem unlikely because other trials of intensive glucose control have similarly shown no improvement in cancer mortality or risk despite larger differences in HbA1c [32]. Finally, because this trial compared two regimens of differing intensities of glucose lowering, it is not possible to examine the effects of individual drugs or classes of drugs on the risk of cancer within the randomised groups.
In conclusion, the randomised data comparing patients assigned to intensive or to standard glucose control who achieved a modest difference in HbA1c of about 0.7% suggest that intensive glucose control achieved with a regimen that included greater use of gliclazide, insulin, metformin and other agents does not affect the risk of cancer in patients with type 2 diabetes over a 5-year follow-up period.
Abbreviations
- ACCORD:
-
Action to Control Cardiovascular Risk in Diabetes
- ADVANCE:
-
Action in Diabetes and Vascular Disease: Preterax and Diamicron-MR Controlled Evaluation
- ICD:
-
International Classification of Diseases
- PY:
-
Person-years
- UKPDS:
-
UK Prospective Diabetes Study
- VADT:
-
Veterans Affairs Diabetes Trial
References
Vigneri P, Frasca F, Sciacca L, Pandini G, Vigneri R (2009) Diabetes and cancer. Endocr Relat Cancer 16:1103–1123
Giovannucci E, Michaud D (2007) The role of obesity and related metabolic disturbances in cancers of the colon, prostate, and pancreas. Gastroenterology 132:2208–2225
Samani AA, Yakar S, LeRoith D, Brodt P (2007) The role of the IGF system in cancer growth and metastasis: overview and recent insights. Endocr Rev 28:20–47
Lam EK, Batty GD, Huxley RR et al (2010) Associations of diabetes mellitus with site-specific cancer mortality in the Asia-Pacific region. Ann Oncol. doi:10.1093/annonc/mdq1405
Stocks T, Rapp K, Bjorge T et al (2009) Blood glucose and risk of incident and fatal cancer in the metabolic syndrome and cancer project (me-can): analysis of six prospective cohorts. PLoS Med 6:e1000201
Stattin P, Bjor O, Ferrari P et al (2007) Prospective study of hyperglycemia and cancer risk. Diabetes Care 30:561–567
Yang X, Ko GT, So WY et al (2010) Associations of hyperglycemia and insulin usage with the risk of cancer in type 2 diabetes: the Hong Kong Diabetes Registry. Diabetes 59:1254–1260
Rapp K, Schroeder J, Klenk J et al (2006) Fasting blood glucose and cancer risk in a cohort of more than 140,000 adults in Austria. Diabetologia 49:945–952
Saydah SH, Platz EA, Rifai N, Pollak MN, Brancati FL, Helzlsouer KJ (2003) Association of markers of insulin and glucose control with subsequent colorectal cancer risk. Cancer Epidemiol Biomarkers Prev 12:412–418
Jonasson JM, Ljung R, Talback M, Haglund B, Gudbjornsdottir S, Steineck G (2009) Insulin glargine use and short-term incidence of malignancies—a population-based follow-up study in Sweden. Diabetologia 52:1745–1754
Colhoun HM, SDRN Epidemiology Group (2009) Use of insulin glargine and cancer incidence in Scotland: a study from the Scottish Diabetes Research Network Epidemiology Group. Diabetologia 52:1755–1765, Erratum 52: 2469
Bowker SL, Majumdar SR, Veugelers P, Johnson JA (2006) Increased cancer-related mortality for patients with type 2 diabetes who use sulfonylureas or insulin. Diabetes Care 29:254–258
Currie CJ, Poole CD, Gale EA (2009) The influence of glucose-lowering therapies on cancer risk in type 2 diabetes. Diabetologia 52:1766–1777
Bowker SL, Yasui Y, Veugelers P, Johnson JA (2010) Glucose-lowering agents and cancer mortality rates in type 2 diabetes: assessing effects of time-varying exposure. Diabetologia 53:1631–1637
Smith U, Gale EA (2009) Does diabetes therapy influence the risk of cancer? Diabetologia 52:1699–1708
Gerstein HC (2010) Does insulin therapy promote, reduce, or have a neutral effect on cancers? JAMA 303:446–447
Nagel JM, Mansmann U, Wegscheider K, Rohmel J (2010) Insulin resistance and increased risk for malignant neoplasms: confounding of the data on insulin glargine. Diabetologia 53:206–208
Pocock SJ, Smeeth L (2009) Insulin glargine and malignancy: an unwarranted alarm. Lancet 374:511–513
Garg SK, Skyler JS (2009) Insulin glargine and cancer—an unsubstantiated allegation. Diabetes Technol Ther 11:473–476
Patel A, MacMahon S, Chalmers J et al (2007) Effects of a fixed combination of perindopril and indapamide on macrovascular and microvascular outcomes in patients with type 2 diabetes mellitus (the ADVANCE trial): a randomised controlled trial. Lancet 370:829–840
Patel A, MacMahon S, Chalmers J et al (2008) Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med 358:2560–2572
The ADVANCE Collaborative Group (2001) Study rationale and design of ADVANCE: action in diabetes and vascular disease—preterax and diamicron MR controlled evaluation. Diabetologia 44:1118–1120
Chodick G, Heymann AD, Rosenmann L et al (2010) Diabetes and risk of incident cancer: a large population-based cohort study in Israel. Cancer Causes Control 21:879–887
Inoue M, Iwasaki M, Otani T, Sasazuki S, Noda M, Tsugane S (2006) Diabetes mellitus and the risk of cancer: results from a large-scale population-based cohort study in Japan. Arch Intern Med 166:1871–1877
Kaaks R, Toniolo P, Akhmedkhanov A et al (2000) Serum C-peptide, insulin-like growth factor (IGF)-I, IGF-binding proteins, and colorectal cancer risk in women. J Natl Cancer Inst 92:1592–1600
Toniolo P, Bruning PF, Akhmedkhanov A et al (2000) Serum insulin-like growth factor-I and breast cancer. Int J Cancer 88:828–832
Frasca F, Pandini G, Sciacca L et al (2008) The role of insulin receptors and IGF-I receptors in cancer and other diseases. Arch Physiol Biochem 114:23–37
Okumura M, Yamamoto M, Sakuma H et al (2002) Leptin and high glucose stimulate cell proliferation in MCF-7 human breast cancer cells: reciprocal involvement of PKC-alpha and PPAR expression. Biochim Biophys Acta 1592:107–116
UK Prospective Diabetes Study (UKPDS) Group (1998) Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet 352:837–853
Gerstein HC, Miller ME, Byington RP et al (2008) Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med 358:2545–2559
Duckworth W, Abraira C, Moritz T et al (2009) Glucose control and vascular complications in veterans with type 2 diabetes. N Engl J Med 360:129–139
Johnson JA, Bowker SL (2010) Intensive glycaemic control and cancer risk in type 2 diabetes: a meta-analysis of major trials. Diabetologia 54:25–31
Acknowledgements
This study was performed in collaboration with the Escher project (T6-202), which is a project of the Dutch Top Institute Pharma.
Duality of interest
J. Chalmers, as co-principal investigator for ADVANCE, holds grants from Servier, administered through the University of Sydney. S. Zoungas, A. Patel, M. Woodward, D. E. Grobbee and J. Chalmers have received lecturing fees from Servier. The funding organisations had no role in the design or conduct of the study, collection, management, analysis or interpretation of data or preparation of the manuscript. The ADVANCE Management Committee was responsible for the conduct of the trial and oversaw the approval and submission of the manuscript (the Committee consists of an independent international collaboration of scientists and it has no representatives from either sponsor).
Funding
ADVANCE was funded by grants from Servier and the National Health and Medical Research Council of Australia.
Author information
Authors and Affiliations
Corresponding author
Additional information
An erratum to this article is available at http://dx.doi.org/10.1007/s00125-013-2980-3.
Rights and permissions
About this article
Cite this article
Stefansdottir, G., Zoungas, S., Chalmers, J. et al. Intensive glucose control and risk of cancer in patients with type 2 diabetes. Diabetologia 54, 1608–1614 (2011). https://doi.org/10.1007/s00125-011-2104-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00125-011-2104-x