Abstract
Aims/hypothesis
The support of distal regenerating axons and epidermal nerve fibres through growth factor delivery may depend on the site of delivery. While low-dose systemic insulin provides trophic support for regenerating axons or axons from diabetic animals, its potential action upon the most distal neurites within the epidermis is unknown. In diabetic neuropathy, distal loss of axons is an important clinical and pathological feature. We hypothesised that insulin and IGF-1 delivered intrathecally could support the most distal epidermal nerve fibres.
Materials and methods
As insulin and IGF-1 receptors are present upon sensory ganglion perikarya, we studied the impact of intrathecal delivery of low-dose insulin and equimolar IGF-1 on the density of epidermal axons expressing protein gene product 9.5 in experimental diabetic rats. After 2 months of diabetes induced by streptozotocin injection, intrathecal delivery of low-dose insulin or IGF-1 or saline was provided for 1 month, with comparison to compatible doses of subcutaneous insulin delivery.
Results
Diabetes, in itself, was associated with a decline in epidermal nerve fibre density. Delivery of both intrathecal IGF-1 and insulin was associated with significant improvement in epidermal fibre density (greatest with IGF-1) and length relative to placebo.
Conclusions/interpretation
Central intrathecal delivery of IGF-1 and insulin offers remote support for epidermal nerve fibres, subjected to ’dying-back’ in early diabetic polyneuropathy.
Similar content being viewed by others
Introduction
Very fine unmyelinated nerve fibres innervate the epidermis of the skin for perception of mechanical, nociceptive, thermal, and chemical sensation. In conditions causing a sensory peripheral neuropathy, such as diabetes mellitus, epidermal nerve fibres are depleted and residual axons appear abnormal. Phenotypic changes within residual epidermal nerve fibres may also resemble a human neuropathic pain state.
Previous studies in animal models of nerve injury such as with crush or transection injury have identified an expected loss of downstream terminal epidermal nerve fibres (ENF) [1]. In human diabetes, ENF density (ENFD) and ENF length (ENFL) are reduced [2]. In addition, elevation of noxious thermal and nociceptive thresholds has been correlated with these reductions in ENFD [3]. Some alterations in ENFD have also been conversely associated with thermal hyperalgesia and allodynia resembling human neuropathic pain [4]. Evidence of ENF loss in experimental diabetes provides opportunities to further study its pathophysiological mechanisms.
ENF loss may result from chemotherapy, human immunodeficiency virus infection, and idiopathic causes of sensory neuropathy, as well as from diabetic polyneuropathy [5]. One possible mechanism in the development of ENF loss is a deficiency of neurotrophic support. We have previously demonstrated that intrathecal delivery of insulin, a potent growth factor, promotes regeneration and prevents axonal retrograde loss in a distal sural nerve crush injury through potent actions upon perikaryal insulin receptors found on sensory neurons [6]. We have also demonstrated that intrathecal delivery of low doses of insulin, at levels insufficient to reduce hyperglycaemia, and of equimolar IGF-I were associated with improvement and reversal of motor and sensory conduction velocity slowing in diabetic rats [6]. In addition, insulin and IGF-I both reversed atrophy in myelinated sensory axons within the sural nerve [6].
Previous studies have demonstrated the potential trophic effects upon sensory neurons and their neuritic extensions. Initially, insulin and IGF-I were thought to support the survival of in vitro sensory and sympathetic neurons [7]. A later in vitro study suggested that in cultured adult rat sensory neurones, neurite outgrowth was enhanced by insulin and IGF-I in a dose-dependent manner, but without effects upon neuronal survival [8]. Previous delivery of intrathecal glial-derived neurotrophic factor (GDNF) or neurturin led to promotion of cutaneous axonal growth and branching in a model of diabetes [9].
Although physiological concentrations of insulin enhance in vitro neurite formation through insulin receptor binding, cross-occupancy of IGF-1 receptors occurs at supraphysiological concentrations [10]. In addition, a loss of insulin activity contributes to a secondary partial decline in IGF-I activity [11], which is already diminished in diabetic tissues. As well as with insulin delivery [6], IGF delivery assists in preventing neuropathy in diabetes, even in the face of hyperglycaemia [6, 11].
We therefore used an experimental diabetic rat model to deliver intrathecal growth factors after a latent period of diabetes in order to determine their effect upon epidermal nerve fibres. We hypothesised that remote intrathecal delivery of insulin or IGF-1 would support epidermal nerve fibres in a model of diabetic neuropathy. Our findings suggest that in the setting of experimental diabetes, activation of insulin or IGF-1 receptors grants potent central perikaryal support of terminal epidermal sensory fibres.
Materials and methods
Animals
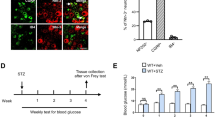
We studied 70 male Sprague–Dawley rats with initial weight of 300 to 350 g. Rats were housed in plastic cages with sawdust-covered floors in a normal light–dark cycle and with free access to rat chow and water. The animals studied were those also studied by Brussee et al. [6]. All protocols were reviewed and approved by the University of Calgary Animal Care Committee using the Canadian Council of Animal Care guidelines. Rats were anaesthetised with pentobarbital (60 mg/kg) prior to all procedures. At the age of 1 month, 40 rats received one intraperitoneal injection of streptozotocin (STZ) 65 mg/kg. The remaining 30 rats were injected once, at identical doses, with citrate buffer vehicle. Following this, all animals had tail blood collected monthly for blood glucose measurements to confirm the presence of diabetes. STZ-injected rats that did not develop diabetes on the basis of a blood glucose threshold of at least 16 mmol/l were excluded from further assessment.
At 8 weeks after STZ or citrate injections, diabetic and non-diabetic control rats were anaesthetised with sodium pentobarbital (65 mg/kg). In 30 STZ-injected animals and all citrate buffer animals, we placed a silicone (0.012′′×0.025′′) catheter, connected to a second silicone catheter (0.025′′×0.047′′), into the lumbar intrathecal space between the L6 and S1 vertebrae. Catheters were connected to a 2-week Alzet infusion pump (Alzet, Cupertino, CA, USA), implanted subcutaneously in the back, as described previously [6]. Identical pumps and catheters have been examined previously and were found to have volume infusion rates of approximately 15 μl per day, or approximately 200 μl over 14 days. As infusion pumps only permitted delivery over a 2-week period, the pump contents were replenished at 2-week intervals, the procedure being performed under sterile conditions and anaesthesia.
All infusion pumps were randomised to contain either insulin (Humulin R; Eli Lilly, Toronto, ON, Canada) or IGF-1 (GroPep, Adelaide, SA, Australia) or saline vehicle in a 1:1:1 ratio. Remaining rats without an intrathecal catheter placement had insulin injected subcutaneously by a similar Alzet pump at a dose of 0.1 IU/day. For infusion pumps containing insulin, delivery of 0.1 IU/day of regular insulin was performed. Infusion pumps containing IGF-1 equimolar to 0.1 IU of insulin were placed in littermates. Fasting blood glucose measurements were performed monthly following electrophysiological testing using the tail vein and a blood glucometer. Separate studies have indicated that intrathecal insulin, at the same dose, does not alter systemic plasma glycaemia [6]. Before STZ injection and growth factor delivery, as well as 4 weeks after treatment, electrophysiological assessment of sciatic nerve conduction parameters was performed [6].
Tissue collection
After an additional 1 month of diabetes, animals were killed by an overdose of pentobarbitol in accordance with the Canadian Council of Animal Care guidelines, and hind foot epithelial footpads were removed in entirety. No punch biopsies were performed. Samples were fixed in 2% Zamboni’s fixative overnight at 4°C, washed in PBS, kept overnight in 20% glucose PBS solution, embedded in optimal cutting temperature embedding solution, and kept at −80°C until sectioning. Using a cryostat, 25-μm sections were prepared and samples were placed onto poly-l-lysine-coated slides.
Tissue analysis
Immunohistochemistry was performed for all cut specimens using an antibody to protein gene product (PGP) 9.5. Slides were incubated with normal goat serum for 1 h at room temperature, then incubated overnight with mouse antiserum against PGP 9.5 (1:500; AbCam, Cambridge, MA, USA) followed by a rinse using PBS. Secondary antibody goat anti-mouse IgG Cy3 (1:100; AbCam) was added to the slides for 1 h at room temperature, and slides were again rinsed with PBS before being cover-slipped with bicarbonate buffered glycerol, and examined under fluorescent microscopy (Axioscope, Axiovision and Axiocam; Zeiss Canada, Toronto, ON, Canada). Fluorescence was examined microscopically using the Cy3 filter (Axioplan 2; Carl Zeiss Canada, Toronto, ON, Canada).
Analysis of the foot pads included quantification of epidermal nerve fibres extending above the dermal papillae per square millimeter of epidermal area. We chose to use this three-dimensional reconstruction due to the obvious advantage of clearly capturing all fibres present within the microsection. In addition to this, we also captured linear densities by calculating the number of fibres within each microsection expressed as a function of epidermal length, which has the disadvantage of calculation of epidermal length over a non-linear surface, as well as of a possible miscounting of fibres within three-dimensional sectioning. A previous study has examined multiple methods of quantifying ENF length and found strong correlational results between three methods, including a third method of nerve fibre estimation based upon the 3-mm size of epidermis biopsy [12]. Nonetheless, the authors of this study concluded that the reporting of nerve fibre profiles per projected surface area is the most useful technique for comparing results from different laboratories due to possible discrepancies in section thickness and shape [12]. However, the use of an epidermal nerve fibre quantification using epidermal length is now considered to be standard [13]. A single Zeiss fluorescent microscope was used in all cases. Digital photography (Zeiss) of all specimen portions was performed and Adobe Photoshop (Adobe Photoshop 7.0, Adobe, San Jose, CA, USA) was used to visualise each specimen for counting purposes.
For each animal, the number of PGP 9.5-immunoreactive profiles was counted by a single observer blinded to the source of each specimen in each of the 25-μm thick microsections, which were performed through the entire footpad. A minimum of 200 microsections was examined in each case. In each case, the area of the epidermal tissue examined was measured by digitalised software to provide a denominator for calculation of nerve fibres per fixed area, with counts expressed as the number of PGP 9.5-positive profiles per square millimeter. In every case, single ENF crossing the dermal-epidermal junction (basement membrane) were counted, whereas secondary branching was not counted. ENF which branched after the basement membrane were not counted as multiple ENF, while those that branched before or at the basement membrane were counted as multiple [14].
ENFD is an estimation of the degree of loss of terminal fibres, probably due to retraction, in diabetic neuropathy [15], and is associated with defined electrophysiological and behavioural abnormalities that also occur in diabetic neuropathy [9]. In addition to ENFD, the ENF length (ENFL) was calculated using Adobe Photoshop software by placing a cursor along the length of each identified ENF. Previous studies have demonstrated that diabetic neuropathy results in shortening and loss of branching in distal-most extremities, suggesting that ENFL is an important measure of diabetic axonal neuropathy and severity of symptoms, and also relates to duration of disease [16].
As noted previously [17], regions within the plantar epidermis were noted to contain PGP 9.5-positive dendritic cells. In order to avoid counting of dendritic cell processes, PGP 9.5-positive profiles seen to be in contact with a cell body likely to be a dendritic cell, were excluded from further consideration. Also, sebaceous glands in the dermis were noted to be PGP 9.5-positive. These, too, were excluded from counting on the basis of morphological differences. Only single epidermal fibres were included in the counts and not secondary branches of individual epidermal fibres. The analysis was performed by an evaluator who was blinded to the nature of treatment of animals that provided the specimens inspected.
For presentation purposes, additional immunohistochemistry was performed using the sections immunostained for PGP 9.5 profile counting. Type IV collagen immunohistochemistry was performed for previously cut specimens. Incubation overnight with rabbit antiserum against collagen type IV (1:100; Chemicon International, Temecula, CA, USA) was followed by a rinse using PBS. Secondary goat anti-rabbit IgG FITC (1:100; Zymed, San Francisco, CA, USA) was added to the slides for 1 h at room temperature, and slides were again rinsed with PBS before being cover-slipped with bicarbonate buffered glycerol, and examined under confocal microscopy (Axiovision; Zeiss). Fluorescence was examined microscopically using the FITC filter (Axioplan 2; Carl Zeiss Canada). For visualisation of melanocytes and Langerhans cells in the epidermis, as well as Schwann cell processes composing nerve sheath in the dermis, S100 immunohistochemistry was performed using sections adjacent to those sections immunostained for collagen type IV. Primary antibody, goat anti-mouse S-100 IgG (1:100; Chemicon), was applied overnight, followed by a rinse using PBS. Secondary antibody goat anti-mouse IgG FITC (1:100; AbCam) was added to the slides for 1 h at room temperature. Fluorescence was examined microscopically using the FITC filter (Axioplan 2; Carl Zeiss). Confocal images were obtained at heights of 1 μm at 20 adjacent levels in order to produce a fused image using Adobe Photoshop (Adobe Photoshop 7.0). A final image was achieved by overlaying confocal images obtained for PGP 9.5 and collagen type IV immunohistochemistry and nonconfocal fluorescent microscopy images for S100.
All data are presented as mean±SEM. Student’s t-testing or ANOVA testing was performed as appropriate in all cases. P values<0.05 were deemed significant.
Results
Diabetes
Of the 40 rats injected with STZ, 28 developed diabetes as determined by a cut-off fasting blood glucose value of 16 mmol/l. Diabetic induction in all of these animals led to uncontrolled hyperglycaemia throughout the study (>16 mmol/l) and a failure to increase weight (<300 g) and volume over the entire study. In contrast, rat weight was >350 g in all control rats. Blood glucose values were higher in diabetic rats, but similar within all intervention groups (intrathecal insulin treatment group, 26.1±3.1; intrathecal IGF-1 treatment group, 31.8±3.4; intrathecal saline group, 23.7±4.5). Non-diabetic rats had euglycaemia when killed (intrathecal insulin, 4.8±2.9; intrathecal saline, 4.6±2.5).
Previously published results using this same cohort of animals demonstrated that the intrathecal delivery of insulin or IGF-I over 1 month in established diabetes improved both motor and sensory conduction abnormalities in diabetic rats. By contrast, cohort diabetic animals had demonstrated expected slowing of both motor and sensory conduction velocities [6]. In addition, intrathecal delivery of insulin or IGF-I prevented sural nerve myelinated fibre axonal atrophy in diabetic rats. There was no difference in dorsal root ganglion neuronal area size between intervention groups [6].
Epidermal foot pads
ENF counts for all individual groups of rodents are displayed in Fig. 1. Non-diabetic rats injected with intraperitoneal citrate buffer carrier had greater numbers of epidermal nerve fibres for each group than their diabetic counterparts. No difference was noted with ENFD between non-diabetic rats implanted with intrathecal pumps delivering saline, insulin, or IGF-1 (Fig. 1). There was also no significant difference in ENFL among non-diabetic rats (Figs. 2 and 3). Diabetic rats had smaller ENFL than control rats for each group. Epidermal nerve fibre densities expressed as a function of epidermal length are provided in Fig. 1. Confocal imaging permitted further identification of epidermal and dermal nerve fibres within individual intervention groups (Fig. 3), again demonstrating an appearance of greater epidermal nerve fibre densities in control rat footpads.
Bar graphs demonstrating the epidermal nerve fibre density within each group of rodents based upon epidermal area (a) or epidermal linear density (b). Diabetes was associated with epidermal fibre loss in all cases. Intrathecal delivery of insulin or IGF-1 was associated with less substantial loss of epidermal nerve fibres *p<0.05 (ANOVA) for loss of epidermal nerve fibre density as compared to any of the three control groups. #p<0.05 (ANOVA) for more epidermal nerve fibres as compared to groups indicated by *. Error bars indicate SEM
Bar graph demonstrating the epidermal nerve fibre length within each group of rodents. Diabetes was associated with epidermal fibre shrinkage in all cases. Intrathecal delivery of insulin or IGF-1 was associated with less substantial loss of size of epidermal nerve fibres. *p<0.05 (ANOVA) for loss of epidermal nerve fibre length as compared to any of the three control groups. #p<0.05 (ANOVA) for greater epidermal nerve fibre length as compared to groups indicated by *. Error bars indicate SEM
Epidermis with epidermal nerve fibres demonstrated in each intervention and control group. Epidermal nerve fibres were identified using PGP 9.5 immunohistochemistry (green). The basement membrane and vasculature is identified using collagen type IV Immunohistochemistry (red). Melanocytes and Langerhans cells in the epidermis as well as Schwann cell sheaths in the dermis are identified with S100 immunohistochemistry (blue). Images are the result of an overlay of confocal microscopy images for PGP 9.5 and collagen type IV with non-confocal fluorescent microscopy for S100. a An example of a control rodent footpad that was subjected to intrathecal saline pump infusion. b–d Diabetic rodent footpads with interventions including intrathecal insulin pump, intrathecal IGF-1 pump, and intrathecal saline pump respectively. Subcutaneous insulin delivery group footpads are not shown. Diabetic epidermal footpads were associated with loss of epidermal nerve fibre density and length, while provision of intrathecal insulin or IGF-1 partially prevented epidermal nerve fibre loss and shrinkage. Note the presence of end bulbs, or axonal swellings, in some of the diabetic rat epidermal sections (arrows). Magnification: 40×; bar=100 μm
Diabetic rats given intrathecal insulin and IGF-1 had increased ENFD and ENFL as compared with diabetic rats receiving intrathecal saline or subcutaneous insulin (Figs. 1 and 2). However, diabetic rats receiving intrathecal insulin or IGF-1 did not have ENFD and ENFL restored to levels demonstrated in non-diabetic rats. There was no difference in ENFD between diabetic animals given intrathecal saline or subcutaneous insulin. There was no significant difference between ENFD and ENFL in rats provided with intrathecal insulin or intrathecal IGF-1 (Figs. 1, 2 and 3).
Discussion
Our results substantiate the impact of intrathecal delivery of neurotrophic growth factors upon diabetic epidermal nerve fibre loss and atrophy. Loss of axonal area and electrophysiological abnormalities within motor and sensory axons in experimental diabetes was also prevented by provision of intrathecal insulin or IGF-1 [6]. Insulin acting alone through receptors abundantly expressed upon both perikarya and axons can support nerve regeneration when given systemically or intrathecally [18]. We have previously shown that FITC-insulin delivered intrathecally can be visualised at the dorsal root ganglia (DRG) neuronal membrane [6]. Dosages of insulin delivered intrathecally or subcutaneously in this study failed to impact on serum glucose, suggesting minimal systemic effect. Although insulin may act locally to reverse features of diabetic neuropathy when applied near the peripheral nerve [19], insulin delivered systemically in this study failed to benefit diabetic epidermal fibres.
The presence of diabetes led to a significant reduction in the number of PGP 9.5-positive fibres, regardless of intervention, when compared to non-diabetic animals. This incomplete prevention of epidermal nerve fibre loss identified in diabetic rats suggests that diabetic neuropathy is a ‘dying-back’ pathophysiological process, beginning in the most distal fibres in the epidermis [20, 21]. We postulate that this ’dying back’ phenomenon of diabetic neuropathy begins with retraction of epidermal nerve fibres and loss of epidermal nerve fibre density, followed later by axonal atrophy in the peripheral nerve and then axonal loss, followed ultimately by DRG neuronal atrophy. This process may depend upon latency between disease onset and time of intervention, as previous studies have identified a partially reversible loss of epidermal nerve fibres after 7 weeks of STZ-induced diabetes in mice [9].
We studied the impact of diabetes and the impact of adding remote perikaryal neurotrophic support by insulin and IGF-1 at the sensory neuronal soma upon ENFD and ENFL. Restoration of ENFD and ENFL occurred in rats receiving intrathecal IGF-1 or insulin, but was incomplete, probably due to the establishment of diabetes prior to therapeutic intervention. We demonstrated that delivery of intrathecal insulin or IGF-1 at the level of the sensory neuron partially prevented distal-most ENF loss and shrinkage. In contrast, subcutaneous delivery of an identical dose of insulin did not prevent ENF loss or shrinkage. Direct delivery of insulin to the sensory neuron appears to be more effective than near nerve insulin delivery in preventing distal remote axonal atrophy and loss of axons following peripheral nerve traumatic injury as well [6].
There are a number of potential mechanisms by which insulin and IGF-1 may exert this preservative benefit upon ENF. In vitro, insulin exerts a direct neuritic outgrowth effect either upon insulin receptors or through cross-activation of IGF-1 receptors, while IGF-1 also cross-reacts with insulin receptors [10, 22, 23]. It has been previously reported that IGF-1 receptors are preferentially expressed by small DRG neurons [24], while insulin receptors were expressed by both small and large DRG neurons in our previous studies [6]. While it is clear that both insulin and IGF-1 stimulate neuritic outgrowth and other cellular responses within the adult sensory neuron, [7, 8, 20], it is not clear that insulin or IGF-1 promotes neuronal survival [7, 8]. At low physiological concentrations, insulin appears to stimulate neuritic outgrowth using insulin receptors, in contrast to supraphysiological doses, when insulin may also bind to IGF-1-receptors (IGF-R) to exert its actions [24]. Within the experimental diabetic DRG, modifications in the cellular expression of IGF-1 occurs, leading to a preferential expression of IGF-1 and IGF-R within small-diameter (<25 μm) DRG diabetic neurons [24]. In this study, it appears that exogenously supplied insulin or IGF-1 at the sensory neuron may have stabilised the function of the sensory neuron, preventing the loss and shrinkage of distal end terminals in the epidermis.
A number of potential signalling pathways are of importance in insulin and IGF-1 maintenance of ENF. In vitro, a continuous application of insulin reverses defective storage and release of CGRP from diabetic enteric neurons, suggesting one potential role in axonal support [25] and a potential pathophysiology for an enhanced regenerative response. It is known that insulin stimulation upregulates protein–tyrosine phosphorylation [26], p42/p44 mitogen-activated protein kinases (MAPK) [26], and extracellular signal-regulated kinase (ERK) through downstream activation of IRS-2 [27]. In addition, physiological levels of insulin and IGFs, in concentrations sufficient to supplement neurite outgrowth, upregulate neurofilament and tubulin mRNA within human neuroblastoma SH-SY5Y cells in vitro [28]. This upregulation supports both neurite maintenance and neurite extension [29]. Other neurotrophic factors at the diabetic DRG may also provide neuronal and axonal support, as intrathecal delivery of neurturin and GDNF has also provided partial restoration of epidermal fibres [9]. In addition to the effects of IGF-1 on sensory neuritic extension, intramuscular nerve sprouting and upregulation of GAP43/B50, a nerve-specific protein important in nerve regrowth, are also dependent upon and massively upregulated in the presence of IGF or insulin activity [30–32]. In our experiment, provision of central IGF-1 and insulin was associated with greater preservation of distal-most epidermal nerve fibres, which could be due to a combination of upregulation of cytoskeletal elements, enhanced CGRP synthesis, and support of signal-regulated processes.
In vitro, insulin’s ability to modulate the inner mitochondrial membrane potential appears to be through activation of the phosphoinositide 3-kinase (PI 3-K) pathway, which stimulates the phosphorylation of Akt and cAMP response element-binding protein CREB [33]. In vitro studies of DRG neurons from insulin-treated diabetic rats have demonstrated correction of diabetes-related depolarisation of the mitochondrial inner membrane [22] involving activation of the PI 3-K pathway, and subsequent downstream activation of Akt and CREB [27, 34]. In vitro, IGF-1 induces Akt phosphorylation via a PI 3-K dependent pathway, and ERK (p42/p44) phosphorylation via a PI 3-K independent pathway [35]. IGF-I may also have an anti-apoptotic effect, as the phosphorylation of forkhead proteins, important mediators of growth factor-stimulated cellular survival, also depends upon PI 3-K and Akt [35]. It is clear now that activation of Akt and MAPK also correlates with sensory neurite elongation and branching [36]. PI 3-K and Akt may be more important for sensory neurite extension and branching than MAPK-dependent signalling, which may, in fact, be inhibitory to sensory neuritic branching [36], although this is controversial [37]. Elegant compartmentalised culture studies have demonstrated that IGF-1 (but neither fibroblast growth factor nor endothelial growth factor) potentiates distal neurite growth into side compartments only when IGF-1 is applied to cell bodies [37]. Clearly, insulin and IGF-1 stabilise the sensory neuron and potentiate its well being following insult or injury.
Small fibre neuropathy in human patients is a major source for disability due to sensory loss and neuropathic pain. In patients with small fibre neuropathy due to diabetes, nerve conduction studies can be normal, and the use of an epidermal punch biopsy may be necessary to identify early distal loss of epidermal axons [38, 39]. Both diabetes and impaired glucose tolerance are associated with a decreased ENFD in humans [16, 40]. In particular, the duration of clinical diabetes has an inverse correlation with ENFD [16, 41]. Clearly, there are similarities between our experimental findings and the human condition, and future studies may lead to potential clinical interventions for patients with diabetic small fibre neuropathy.
The results of this study suggest that loss of insulin or IGF-1 support at the level of the sensory neuron may be crucial in the development of ENF loss and shrinkage. We have also confirmed that when diabetes duration is long enough, the condition is associated with morphological change at the distal end terminals. Further studies will be required to identify the cause of the hypothesised loss of neurotrophic support at the sensory neuron, although the loss of IGF-1 and deficiency of insulin-regulated cell signalling in plasma, DRG and peripheral nerves in diabetic patients may play an important role [11, 20].
Abbreviations
- CGRP:
-
calcitonin gene-related peptide
- CREB:
-
cAMP response element-binding protein
- DRG:
-
dorsal root ganglia
- ENF:
-
epidermal nerve fibres
- ENFD:
-
ENF density
- ENFL:
-
ENF length
- ERK:
-
extracellular signal-regulated kinase
- GDNF:
-
glial-derived neurotrophic factor
- IGF-R:
-
IGF receptor
- MAPK:
-
mitogen-activated protein kinase
- PGP:
-
protein gene product
- PI 3-K:
-
phosphoinositide 3-kinase
- STZ:
-
streptozotocin
References
Navarro X, Verdu E, Wendelschafer-Crabb G, Kennedy WR, (1997) Immunohistochemical study of skin reinnervation by regenerative axons. J Comp Neurol 380:164–174
Hirai A, Yasuda H, Joko M, Maeda T, Kikkawa R (2000) Evaluation of diabetic neuropathy through the quantitation of cutaneous nerves. J Neurol Sci 172:55–62
Bergmann I, Priestley JV, McMahon SB, Brocker EB, Toyka KV, Koltzenburg M (1997) Analysis of cutaneous sensory neurons in transgenic mice lacking the low affinity neurotrophin receptor p75. Eur J Neurosci 9:18–28
Lindenlaub T, Sommer C (2002) Epidermal innervation density after partial sciatic nerve lesion and pain-related behavior in the rat. Acta Neuropathol (Berl) 104:137–143
McArthur JC, Stocks EA, Hauer P, Cornblath DR, Griffin JW (1998) Epidermal nerve fiber density: normative reference range and diagnostic efficiency. Arch Neurol 55:1513–1520
Brussee V, Cunningham FA, Zochodne DW (2004) Direct insulin signaling of neurons reverses diabetic neuropathy. Diabetes 53:1824–1830
Recio-Pinto E, Rechler MM, Ishii DN (1986) Effects of insulin, insulin-like growth factor-II, and nerve growth factor on neurite formation and survival in cultured sympathetic and sensory neurons. J Neurosci 6:1211–1219
Fernyhough P, Willars GB, Lindsay RM, Tomlinson DR (1993) Insulin and insulin-like growth factor I enhance regeneration in cultured adult rat sensory neurones. Brain Res 607:117–124
Christianson JA, Riekhof JT, Wright DE (2003) Restorative effects of neurotrophin treatment on diabetes-induced cutaneous axon loss in mice. Exp Neurol 179:188–199
Recio-Pinto E, Ishii DN (1988) Insulin and insulin-like growth factor receptors regulating neurite formation in cultured human neuroblastoma cells. J Neurosci Res 19:312–320
Ishii DN (1995) Implication of insulin-like growth factors in the pathogenesis of diabetic neuropathy. Brain Res Brain Res Rev 20:47–67
Hilliges M, Johansson O (1999) Comparative analysis of numerical estimation methods of epithelial nerve fibers using tissue sections. J Peripher Nerv Syst 4:53–57
Lauria G, Cornblath DR, Johansson O et al (2005) EFNS guidelines on the use of skin biopsy in the diagnosis of peripheral neuropathy. Eur J Neurol 12:747–758
Kennedy WR, Wendelschafer-Crabb G, Polydefkis M, McArthur J (2005) Pathology and quantitation of cutaneous nerves. In: Dyck PJ, Thomas PK (eds) Pathology and quantitation of cutaneous nerves, 4th edn. Saunders, Philadelphia, pp 869–896
Brismar K, Fernqvist-Forbes E, Wahren J, Hall K (1994) Effect of insulin on the hepatic production of insulin-like growth factor-binding protein-1 (IGFBP-1), IGFBP-3, and IGF-I in insulin-dependent diabetes. J Clin Endocrinol Metab 79:872–878
Pittenger GL, Ray M, Burcus NI, McNulty P, Basta B, Vinik AI (2004) Intraepidermal nerve fibers are indicators of small-fiber neuropathy in both diabetic and nondiabetic patients. Diabetes Care 27:1974–1979
Stankovic N, Johansson O, Hildebrand C (1996) Occurrence of epidermal nerve endings in glabrous and hairy skin of the rat foot after sciatic nerve regeneration. Cell Tissue Res 284:161–166
Xu QG, Li XQ, Kotecha SA, Cheng C, Sun HS, Zochodne DW (2004) Insulin as an in vivo growth factor. Exp.Neurol. 188:43–51
Singhal A, Cheng C, Sun H, Zochodne DW (1997) Near nerve local insulin prevents conduction slowing in experimental diabetes. Brain Res 763:209–214
Toth C, Brussee V, Cheng C, Zochodne DW (2004) Diabetes mellitus and the sensory neuron. J Neuropathol Exp Neurol 63:561–573
Zochodne DW, Verge VM, Cheng C, Sun H, Johnston J (2001) Does diabetes target ganglion neurones? Progressive sensory neurone involvement in long-term experimental diabetes. Brain 124:2319–2334
Huang TJ, Price SA, Chilton L et al (2003) Insulin prevents depolarization of the mitochondrial inner membrane in sensory neurons of type 1 diabetic rats in the presence of sustained hyperglycemia. Diabetes 52:2129–2136
Edbladh M, Fex-Svenningsen A, Ekstrom PA, Edstrom A (1994) Insulin and IGF-II, but not IGF-I, stimulate the in vitro regeneration of adult frog sciatic sensory axons. Brain Res 641:76–82
Craner MJ, Klein JP, Black JA, Waxman SG (2002) Preferential expression of IGF-1 in small DRG neurons and down-regulation following injury. Neuroreport 13:1649–1652
Burnstock G, Mirsky R, Belai A (1988) Reversal of nerve damage in streptozotocin-diabetic rats by acute application of insulin in vitro. Clin Sci (Lond) 75:629–635
Mahadev K, Wu X, Motoshima H, Goldstein BJ (2004) Integration of multiple downstream signals determines the net effect of insulin on MAP kinase vs. PI 3′-kinase activation: potential role of insulin-stimulated H(2)O(2). Cell Signal 16:323–331
Huang C, Thirone AC, Huang X, Klip A (2005) Differential contribution of insulin receptor substrates 1 versus 2 to insulin signaling and glucose uptake in l6 myotubes. J Biol Chem 280:19426–19435
Wang C, Li Y, Wible B, Angelides KJ, Ishii DN (1992) Effects of insulin and insulin-like growth factors on neurofilament mRNA and tubulin mRNA content in human neuroblastoma SH-SY5Y cells. Brain Res Mol Brain Res 13:289–300
Fernyhough P, Mill JF, Roberts JL, Ishii DN (1989) Stabilization of tubulin mRNAs by insulin and insulin-like growth factor I during neurite formation. Brain Res Mol Brain Res 6:109–120
Caroni P, Grandes P (1990) Nerve sprouting in innervated adult skeletal muscle induced by exposure to elevated levels of insulin-like growth factors. J Cell Biol 110:1307–1317
Caroni P, Schneider C, Kiefer MC, Zapf J (1994) Role of muscle insulin-like growth factors in nerve sprouting: suppression of terminal sprouting in paralyzed muscle by IGF-binding protein J Cell Biol 125:893–902
Caroni P, Becker M (1992) The downregulation of growth-associated proteins in motoneurons at the onset of synapse elimination is controlled by muscle activity and IGF1. J Neurosci 12:3849–3861
Huang TJ, Verkhratsky A, Fernyhough P (2005) Insulin enhances mitochondrial inner membrane potential and increases ATP levels through phosphoinositide 3-kinase in adult sensory neurons. Mol Cell Neurosci 28:42–54
Fernyhough P, Huang TJ, Verkhratsky A (2003) Mechanism of mitochondrial dysfunction in diabetic sensory neuropathy. J Peripher Nerv Syst 8:227–235
Schwab TS, Madison BB, Grauman AR, Feldman EL (2005) Insulin-like growth factor-I induces the phosphorylation and nuclear exclusion of forkhead transcription factors in human neuroblastoma cells. Apoptosis 10:831–840
Jones DM, Tucker BA, Rahimtula M, Mearow KM (2003) The synergistic effects of NGF and IGF-1 on neurite growth in adult sensory neurons: convergence on the PI 3-kinase signaling pathway. J Neurochem 86:1116–1128
Kimpinski K, Mearow K (2001) Neurite growth promotion by nerve growth factor and insulin-like growth factor-1 in cultured adult sensory neurons: role of phosphoinositide 3-kinase and mitogen activated protein kinase. J Neurosci Res 63:486–499
Lacomis D (2002) Small-fiber neuropathy. Muscle Nerve 26:173–188
Quattrini C, Jeziorska M, Malik RA (2004) Small fiber neuropathy in diabetes: clinical consequence and assessment Int J Low Extrem Wounds 3:16–21
Sumner CJ, Sheth S, Griffin JW, Cornblath DR, Polydefkis M (2003) The spectrum of neuropathy in diabetes and impaired glucose tolerance Neurology 60:108–111
Shun CT, Chang YC, Wu HP et al (2004) Skin denervation in type 2 diabetes: correlations with diabetic duration and functional impairments. Brain 127:1593–1605
Acknowledgements
This study was supported by an operating grant from the Canadian Institutes of Health Research (CIHR) and the Canadian Diabetes Association (CDA). C. Toth was a Clinical Fellow of the Alberta Heritage Foundation for Medical Research and D. W. Zochodne is a Scientist of Alberta Heritage Foundation for Medical Research.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Toth, C., Brussee, V. & Zochodne, D.W. Remote neurotrophic support of epidermal nerve fibres in experimental diabetes. Diabetologia 49, 1081–1088 (2006). https://doi.org/10.1007/s00125-006-0169-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00125-006-0169-8