Abstract
Aims/hypothesis
One in four children with Type 1 diabetes in a population-based family study has an affected grandparent. We set out to study the clinical and immune features of diabetes in the grandparents' generation, and to examine sharing of HLA class II susceptibility haplotypes between grandparent and grandchild.
Methods
Of 5855 grandparents in the Bart's-Oxford family study, 428 (7.3%) were known to have diabetes. Clinical data and samples were collected from 115 of 213 surviving affected grandparents and from 219 unaffected grandparents within the same families. Samples were tested for ICA and autoantibodies to GAD and IA-2, and typed for HLA-DRB1-DQA1-DQB1. Transmission of HLA class II haplotype from affected and unaffected grandparents to the diabetic proband was compared.
Results
Of 115 affected grandparents studied, the median age at diagnosis was 61 years and at analysis was 73 years; 70% were diet or tablet treated and 30% were on insulin. One or more islet autoantibodies were found in 26% and 66% had one or both of the high risk HLA class II susceptibility haplotypes DRB1*03-DQA1*0501-DQB1*0201 or DRB1*04-DQA1*0301-DQB1*0302. In 79 informative families the HLA class II haplotype of the affected grandparent was transmitted to the proband more frequently than expected overall (59%, p=0.02), and in the insulin-treated subgroups (65%, p=0.03).
Conclusion/interpretation
A total of 7.3% of grandparents reported a clinical diagnosis of diabetes and 2.2% had features of Type 1 diabetes. Genetic susceptibility was shared between grandparents with diabetes and their affected grandchildren. Diabetes in the grandparents of children with Type 1 diabetes often has an autoimmune basis, even when it presents late in life and does not require insulin treatment.
Similar content being viewed by others
Immune-mediated diabetes is not exclusively, or even typically, a disease of childhood. Presentation in later life ranges across a spectrum; at one end are those who present acutely and require immediate insulin treatment, and at the other are those who present as Type 2 diabetes yet carry islet autoantibodies. Since the classification of immune-mediated diabetes was previously based solely upon an early requirement for insulin [1], the frequency of the condition could have been underestimated in older relatives of children with classic Type 1 diabetes. With this in mind, we examined the parents of children with Type 1 diabetes in an earlier study, and found that 3.1% had insulin-treated diabetes and 2.1% did not require insulin. When aetiological criteria were used, however, we found that 4.5% had immune-mediated diabetes and only 0.9% had a non-immune form of the disease; the latter estimate did not differ from reported rates in the background population [2].
A prolonged prodromal period with onset in later life is characteristic of many other autoimmune conditions, and it would not be surprising if a proportion of cases of Type 1 diabetes presented in this way; this may even have been the characteristic mode of onset in earlier generations. On this basis, we have speculated that a more permissive environment might lead to earlier clinical presentation in genetically predisposed subjects, and that earlier disease onset might partially explain the rising incidence of childhood onset Type 1 diabetes over the past 50 years [3, 4]. Another example of earlier disease onset in response to a more permissive environment would be the current epidemic of obesity-related childhood onset Type 2 diabetes [5]. We therefore aimed to establish whether examination of the grandparental generation would add biological support to this concept, and set out to determine the prevalence of immune-mediated diabetes in this generation, and to look for evidence of shared genetic susceptibility between affected grandparent and grandchild.
Subjects and methods
Grandparents with diabetes were identified through the families participating in the Bart's Oxford (BOX) study [6]. The BOX study is an ongoing prospective population based family study in the former Oxford Regional Health Authority area. Since it began in 1985, the study has recruited 88% of the families of children diagnosed with Type 1 diabetes below the age of 21. By the end of December 2001, 1693 families had been recruited into the study with 89% under regular follow up. A family history of diabetes in first and second degree relatives of the affected child was collected at study entry.
The diabetes status of the grandparents was updated annually by a questionnaire in all families under follow up. Families in which one or more grandparent was known to have diabetes were contacted to ascertain the health status of both affected and unaffected grandparents. They were also asked to provide contact details for all surviving grandparents if relatives thought this appropriate. Grandparents were sent an explanatory letter about the BOX study and were invited to join. Unaffected grandparents in these families were also recruited. A single visit was arranged at which detailed information about diagnosis of diabetes and time to insulin treatment were recorded. A medical history was obtained in all grandparents and included specific details about other autoimmune diseases. Grandparents were asked to provide blood samples for autoantibody assessment and genetic analysis, and those without diabetes were also invited to give a sample for HbA1c analysis. Four individuals who did not wish to give blood were invited to provide a mouth swab for genotyping. Islet cell antibodies (ICA) and antibodies to glutamic acid decarboxylase (GAD) and the protein tyrosine phosphatase IA-2 were assessed in the remainder, and genotyping for HLA-DRB1, DQA1 and DQB1 alleles was carried out in all cases.
General practitioners of unaffected grandparents who had an HbA1c value above 6% were contacted and asked to carry out further screening for diabetes.
Informed consent was obtained from all study subjects. The study was approved by the ethics committees of all centres involved in the study and was carried out in accordance with the Declaration of Helsinki as revised in 2000.
Assays
ICA were measured in sera by indirect immunofluorescence as described previously [7]. End point titres were converted to Juvenile Diabetes Foundation (JDF) units by comparison with a standard curve of log2 JDF units versus log2 of the end point titre of the standard sera. The threshold of ICA detection was 5 JDF units. Antibodies to GAD65 and IA-2ic were measured by radioimmunoassay [7]. The ICA assay achieved 81% sensitivity with 86% specificity, the GAD antibody assay 91% sensitivity with 99% specificity, and the IA-2 antibody assay 74% sensitivity with 99% specificity in the first Immunology of Diabetes Society (IDS) combined antibody workshop [8].
HLA genotyping was carried out on DNA from blood or mouth swab samples. Details of DNA extraction methods and HLA class II analysis have been published [9]. Briefly, mouth swab extractions were carried out using a guanidium chloride/phenol:chloroform method. Low yield DNA samples from mouth swabs underwent whole genome amplification by primer extension preamplification where necessary. HLA analysis was carried out by polymerase chain reaction using sequence specific primers [10]. Samples were typed for HLA-DRB1*01–10, for DQA1*0201, 0301, 0302 and 0501, and for subtypes of DQB1*02–06. Extended HLA genotypes were constructed. Subjects who did not have the high risk haplotypes DRB1*03-DQA1*0501-DQB1*0201 or DRB1*04-DQA1*0301-DQB1*0302 or the protective haplotype DRB1*02-DQB1*0602 were reported as DRX.
HbA1c was measured by high pressure liquid chromatography (HPLC) using a HAH140 analyser (BioMen Diagnostics, Falcon Business Park, Finchampstead, UK).
Data analysis
Classification of diabetes in affected grandparents was based on current treatment, time from diagnosis to insulin treatment and the presence or absence of one or more islet cell autoantibodies. Grandparents were classified as having Type 1 diabetes if permanent insulin therapy was started within 12 months of diagnosis and/or one or more islet-cell autoantibody was present. Affected grandparents were considered to have Type 2 diabetes if they did not require insulin and were antibody negative.
Autoantibody analyses for ICA, GAD and IA-2 were considered positive when above the 97.5th centile of a control population of schoolchildren [7]. This corresponded to 5 JDF units for ICA, 1.6 units for GAD antibodies and 0.9 units for IA-2 antibodies.
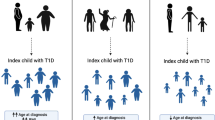
HLA DRB1-DQA1-DQB1 genotype was analysed in the proband, parents and affected and unaffected grandparent to study transmission of all typed HLA haplotypes through the three generations. The frequency of transmission of the HLA haplotype from the affected grandparent to the proband was calculated and compared by Chi squared testing (Fig. 1). A p value of less than 0.05 was considered significant.
Transmission of the HLA haplotype from affected grandparent through the parent to the proband. Grandparents or grandchildren affected with diabetes are shaded. The parental generation does not necessarily have diabetes but carries and transmits the disease-linked HLA haplotype to the next generation. The expected transmission of grandparental haplotypes from parent to proband is 50%
Results
Grandparents known to have diabetes
One or more grandparent had a diagnosis of diabetes in 369 of 1500 families (24.6%) under follow up. In total 428 (7.3%) of 5855 grandparents were known to have diabetes, one grandparent was affected in 315 families, two grandparents in 49 families and three grandparents in five families. Of 213 surviving grandparents, 115 were willing and able to provide further information and samples (Fig. 2). The characteristics of the study group matched those of the whole group of grandparents with diabetes on whom treatment details were available (Table 1).
The median age of the affected group at the time of study was 73 years, range 46 to 89 years. Age at onset of diabetes ranged from 7 to 81, median 61 years. The overall male to female ratio in the affected group was 1:1.05, 30% were treated with insulin and time to insulin from diagnosis varied from 0 to 25 years. The immunogenetic features of the subgroup studied are shown in Table 2. Autoantibody analysis was carried out in 111 affected grandparents, as four did not give a blood sample. One or more islet autoantibodies were present in 26% and 8% had two or more; 14% had ICA, median 10.5 JDF units, range 7 to over 80; 18% GAD antibodies, median value 17 units, range 2 to over 100; and 3% IA-2 antibodies, median 3, range 1.5 to 3.2. Samples were taken from 10 months to 48 years after diagnosis. The highest risk HLA genotype DRB1*03-DQA1*0501-DQB1*0201 / DRB1*04-DQA1*0301-DQB1*0302 was present in 9% of the 115 affected grandparents, 66% had either one or both of the above haplotypes and 15% had the protective HLA haplotype DQB1*0602.
Of the 115 affected grandparents assessed, 36 were classified as having Type 1 diabetes (Fig. 3). Seven of these had started insulin treatment within 12 months of diagnosis, 20 had one or more islet autoantibody, and 9 fulfilled both criteria. Median age at diagnosis in the Type 1 diabetes group was 58, range 7 to 77 years. In those who started insulin early this was 52 (range 7–75 years), and was 63 (range 52–77 years) in those with antibodies but not on insulin. There were 62 grandparents who were both antibody negative and not insulin-treated, and these were considered to have Type 2 diabetes. Median age at diagnosis in this group was 68, range 32 to 81 years. No classification was made in 17, including 4 who had not provided blood samples for antibody estimation, and 13 who started insulin more than 12 months after diagnosis and were antibody negative 2 to 35 years after disease onset. The highest risk HLA genotype DRB1*03-DQA1*0501-DQB1*0201 / DRB1*04-DQA1*0301-DQB1*0302 was present in 17% of the 36 grandparents with Type 1 diabetes and 5% of the 62 with Type 2 diabetes; 66% and 69% respectively had either one or both of the above haplotypes and 6% and 18% respectively had the protective HLA haplotype DQB1*0602.
Basis of classification of Type 1 diabetes in grandparents. Of the 115 grandparents studied, 79 had either Type 2 diabetes (n=62) or were classified as indeterminate (n=17), and 36 had Type 1 diabetes. Of these seven were insulin treated within 12 months of diagnosis, in 20 one or more islet autoantibody above the 97.5th centile was present and both factors were found in nine
We were able to extrapolate our findings from the subgroup analysed to the grandparents as a whole, since the baseline features were similar (Table 1). Assessment of the 115 grandparents led to the classification of 36 (31.3%) as having Type 1 diabetes. The number of grandparents with Type 1 diabetes from the group as a whole can then be estimated as 129, that is 31.3% of the 413 grandparents with treatment details. Thus 129 of 5855 or 2.2% of grandparents of a child with Type 1 diabetes could be estimated as having Type 1 diabetes.
Grandparents not known to have diabetes
Information and blood or mouth swab samples were provided by 219 grandparents not known to have diabetes. Median age at study entry was 71 years, range 47 to 93. One or more islet antibodies were present in 15% of the 211 blood samples collected and two or more in 2%; 10% ICA, median 10 JDF units, range 7 to over 80; 6% had GAD antibodies, median value 5.2 units, range 1.6 to 96; and 1.4% IA-2 antibodies, median 1.2 units, range 1.0 to 1.8. HbA1c was above 7% in 3% of the samples measured, one antibody was increased in only one of these subjects. The highest risk HLA genotype DRB1*03-DQA1*0501-DQB1*0201 / DRB1*04-DQA1*0301-DQB1*0302 was present in 6% of the 210 unaffected grandparents who had provided a genetic sample, 65% had either one or both of the above haplotypes and 15% had the protective HLA haplotype DQB1*0602.
Transmission of HLA class II haplotypes
Eighty-three grandparents (from 79 families) were assessed for HLA DRB1-DQA1-DQB1 haplotype transmission. Families where both grandparents had diabetes and those with uninformative transmission were excluded from the analysis. Parents transmitted the haplotype they had received from the affected grandparent to their affected child in 59% of the families rather than 50% as expected (p=0.02). Transmission was analysed according to the treatment of the affected grandparent: insulin treated (n=26), tablet treated (n=40) and diet alone (n=17). Parents transmitted the haplotype from the affected grandparent in 65% of the insulin group (p=0.027), 60% of the tablet group (p=0.07) and 47% of the diet treated group (p=0.7).
Discussion
We found that 7.3% of grandparents of children with Type 1 diabetes also had a diagnosis of diabetes. This estimate should be viewed with some caution. One potential source of bias lies in the fact that many of the grandparents had either died or were unavailable for study. The subgroup tested did however resemble the larger group of subjects who could not be studied. A second concern is that many reportedly non-diabetic grandparents are likely to have undiagnosed diabetes, since this is common in this age group [11, 12]. We attempted to evaluate the extent of this problem by measuring HbA1c in the control group of unaffected grandparents recruited for genetic analysis. The biological variance of HbA1c is however such that it has limited value as a screening test for diabetes [13], and we encountered a high proportion of moderately increased values which were difficult to evaluate. The estimates we have used therefore exclude previously unrecognised diabetes, and under-represent the true prevalence of diabetes in the grandparental generation. However epidemiological surveys in United Kingdom populations found a somewhat lower prevalence of known diabetes (4.6–5.3%) across a similar age group [14, 15].
Using the classification scheme outlined above, 2.2% had features of Type 1 diabetes. Our classification scheme was based upon early requirement for insulin, defined as insulin treatment within 12 months of diagnosis, or islet autoantibodies. Childhood onset Type 1 diabetes typically presents with the full range of islet autoantibodies [7, 16, 17, 18], but ICA and GAD antibodies are characteristic of late onset immune-mediated diabetes [19]. ICA diminish over time but GAD antibodies persist for many years after diagnosis [20, 21, 22]. We used the presence of these autoantibodies to identify immune-mediated diabetes in grandparents who did not require insulin or had a delayed requirement for it. HLA haplotypes conferring increased susceptibility to Type 1 diabetes can be used to support this diagnosis in other populations, but there will inevitably be an ascertainment bias in the predecessors of affected children. The presence of high risk haplotypes in affected grandparents is therefore difficult to interpret and we have excluded this criterion from our classification scheme. However the observation that 66% had a high risk haplotype implies that a 2.2% prevalence of immune-mediated diabetes could well be an underestimate in this generation. Since population studies of late onset diabetes have typically allocated diabetes type on the basis of treatment rather than measurement of immune markers, we have no comparable estimate of the rate of immune-mediated diabetes in the background population.
We went on to look at transmission of HLA haplotypes from affected grandparents to the proband. A parent receives one HLA haplotype from each grandparent, and has a 50% chance of transmitting each haplotype to his or her offspring. We found, however, that parents transmitted the haplotype from the affected grandparent to the affected grandchild more often than expected (p=0.02). When the grandparents were sub-divided according to treatment group, the transmission rate of HLA haplotypes from affected grandparents was different from that expected in the insulin group (65%, p=0.027) and approached significance in the tablet-treated group (60% p=0.07) but was at the expected rate in the diet-treated group (47% p=0.7). This is the only study to have assessed transmission of Type 1 diabetes across three generations, and even in this large family study the number of available and informative families remains below 100. Despite this limitation, the increased frequency of haplotype transmission between affected generations suggests that HLA class II susceptibility is shared between grandparents with diabetes and their affected grandchildren.
The clinical and immune features of Type 1 diabetes are therefore over-represented in grandparents of affected children, as compared with the general population. Although the grandchildren presented with classic Type 1 diabetes, its characteristic features were often absent in grandparents, who presented later in life and did not necessarily have an early requirement for insulin. Evidence of increased haplotype sharing strengthens the suggestion that grandparent and grandchild are affected by the same disease process, despite the difference in presentation.
In conclusion, this study has confirmed that treatment-based classification will underestimate the frequency of autoimmune diabetes in family members. It suggests that autoimmune diabetes is over-represented in grandparents of children with Type 1 diabetes, as compared with the general population, and shows that they are more likely to transmit HLA haplotypes to their affected grandchildren. These observations would be consistent with the suggestion that the increasing incidence of childhood onset autoimmune diabetes in the second half of the 20th century represents earlier manifestation of a condition that affected older subjects in previous generations [23]. If so, this would help to explain the persistence of an otherwise lethal trait.
Abbreviations
- BOX:
-
Bart's-Oxford study of childhood diabetes
- ICA:
-
islet cell autoantibodies
Reference
Alberti KGMM, Zimmet PZ (1998) Definition, diagnosis and classification of diabetes mellitus and its complications part 1: diagnosis and classification of diabetes mellitus. Provisional report of a WHO consultation. Diabet Med 15:539–553
Douek IF, Gillespie KM, Bingley PJ, Gale EAM (2002) Diabetes in the parents of children with type 1 diabetes. Diabetologia 45:495–501
Onkamo P, Väänänen S, Karvonen M, Tuomilehto J (1999) Worldwide increase in incidence of Type 1 diabetes—the analysis of the data on published incidence trends. Diabetologia 42:1395–1403
EURODIAB ACE Study Group (2000) Variation and trends in incidence of childhood diabetes in europe. Lancet 355:873–876
Fagot-Campagna A, Narayan KMV, Imperatore G (2001) Type 2 diabetes in children. BMJ 322:377–378
Bingley PJ, Gale EAM (1989) Incidence of insulin dependent diabetes in England: a study in the Oxford region, 1985–1986. BMJ 298:558–560
Bingley PJ, Bonifacio E, Williams AJK, Genovese S, Bottazzo GF, Gale EAM (1997) Prediction of IDDM in the General Population. Strategies based on combinations of autoantibody markers. Diabetes 46:1701–1710
Verge CF, Stenger D, Bonifacio E et al. (1998) Combined use of autoantibodies (IA-2 autoantibody, GAD autoantibody, insulin autoantibody, cytoplasmic islet cell antibodies) in type 1 diabetes. Combinatorial islet autoantibody workshop. Diabetes 47:1857–1866
Gillespie KM, Valovin SJ, Saunby J et al. (2000) HLA class II typing of whole genome amplified mouth swab DNA. Tissue Antigens 56:530–538
Bunce M, O'Neill CM, Barnado MCNM, Krausa P, Morris PJ, Welsh KI (1995) Phototyping: comprehensive DNA typing for HLA-A, B, C, DRB1, DRB3, DRB4, DRB5 and DQB1 by PCR with 144 primer mixes utilising sequence-specific primers (PCR-SSP). Tissue Antigens 46:355–367
Lawrence JM, Bennett P, Young A, Robinson AM (2001) Screening for diabetes in general practice: cross sectional population study. BMJ 323:548–551
Croxson SC, Burden AC, Bodington M, Botha JL (1991) The prevalence of diabetes in elderly people. Diabet Med 8:28–31
Kilpatrick ES, Maylor PW, Keevil BG (1998) Biological variance of glycated hemoglobin. Diabetes Care 21:261–264
Gatling W, Budd S, Walters D, Mullee MA, Goddard JR, Hill RD (1998) Evidence of an increasing prevalence of diagnosed diabetes mellitus in the Poole area from 1983 to 1996. Diabet Med 15:1015–1021
Morris AD, Boyle DIR, MacAlpine R et al. (1997) The diabetes audit and research in Tayside Scotland (DARTS) study: electronic record linkage to create a diabetes register. BMJ 315:524–528
Verge CF, Howard NJ, Rowley MJ et al. (1994) Anti-glutamate decarboxylase and other antibodies at the onset of childhood IDDM: a population based study. Diabetologia 37:1113–1120
Vandewalle CL, Falorni A, Svanholm S et al. (1995) High diagnostic sensitivity of glutamate decarboxylase autoantibodies in insulin-dependent diabetes mellitus with clinical onset between age 20 and 40 years. J Clin Endocrinol Metab 80:846–851
Gorus FK, Goubert P, Semakula C et al. (1997) IA-2-autoantibodies complement GAD65-autoantibodies in new-onset IDDM patients and help predict impending diabetes in their siblings. Diabetologia 40:95–99
Li H, Isomaa B, Taskinen MR, Groop L, Tuomi T (2000) Consequences of a family history of type 1 and type 2 diabetes on the phenotype of patients with type 2 diabetes. Diabetes Care 23:589–594
Savola K, Sabbah E, Kulmala P, Vähäsalo P, Ilonen J, Knip M (1998) Autoantibodies associated with type 1 diabetes mellitus persist after diagnosis in children. Diabetologia 41:1293–1297
Decochez K, Tits J, Coolens JL et al. (2000) High frequency of persisting or increasing islet-specific autoantibody levels after diagnosis of type 1 diabetes presenting before 40 years of age. Diabetes Care 23:838–844
Dromey JA, Mijovic CH, Christie MR et al. (2000) HLA linked persistence of antibodies to GAD 65 in patients with 50 years of type 1 diabetes. Diabet Med 17: A73
Gale EAM (2002) The rise of childhood Type 1 diabetes in the twentieth century. Diabetes 51:3353–3361
Acknowledgements
This study was supported by Diabetes UK. We acknowledge the continuing help and support of the children and their families; of the study fieldworkers; and of paediatricians, physicians, and diabetes nurse specialists in the Oxford region. We also wish to thank the project administrators, Isabel Wilson and Suzanne Weeks and the BOX study fieldworkers for all their help. We are grateful to A. Williams and A. Norcross for their technical assistance.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Douek, I.F., Gillespie, K.M., Dix, R.J. et al. Three generations of autoimmune diabetes: an extended family study. Diabetologia 46, 1313–1318 (2003). https://doi.org/10.1007/s00125-003-1186-5
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00125-003-1186-5