Abstract
Key message
This paper reports fine mapping of qCLS for resistance to Cercospora leaf spot disease in mungbean and identified LOC106765332encoding TATA-binding-protein-associated factor 5 (TAF5) as the candidate gene for the resistance
Abstract
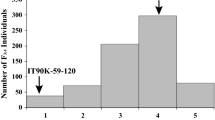
Cercospora leaf spot (CLS) caused by the fungus Cercospora canescens is an important disease of mungbean. A QTL mapping using mungbean F2 and BC1F1 populations developed from the “V4718” (resistant) and “Kamphaeng Saen 1” (KPS1; susceptible) has identified a major QTL controlling CLS resistance (qCLS). In this study, we finely mapped the qCLS and identified candidate genes at this locus. A BC8F2 [KPS1 × (KPS1 × V4718)] population developed in this study and the F2 (KPS1 × V4718) population used in a previous study were genotyped with 16 newly developed SSR markers. QTL analysis in the BC8F2 and F2 populations consistently showed that the qCLS was mapped to a genomic region of ~ 13 Kb on chromosome 6, which contains only one annotated gene, LOC106765332 (designated “VrTAF5”), encoding TATA-binding-protein-associated factor 5 (TAF5), a subunit of transcription initiation factor IID and Spt-Ada-Gcn5 acetyltransferase complexes. Sequence comparison of VrTAF5 between KPS1 and V4718 revealed many single nucleotide polymorphisms (SNPs) and inserts/deletions (InDels) in which eight SNPs presented in eight different exons, and an SNP (G4,932C) residing in exon 8 causes amino acid change (S250T) in V4718. An InDel marker was developed to detect a 24-bp InDel polymorphism in VrTAF5 between KPS1 and V4718. Analysis by RT-qPCR showed that expression levels of VrTAF5 in KPS1 and V4718 were not statistically different. These results indicated that mutation in VrTAF5 causing an amino acid change in the VrTAF5 protein is responsible for CLS resistance in V4718.








Similar content being viewed by others
Availability of data and materials
All information is specified in the manuscript or included as Additional Files.
References
AVRDC (1974) AVRDC progress report 1974. Asian Vegetable Research and Development Center, Shanhua, Taiwan, China
AVRDC (1976) AVRDC progress report 1975. Asian Vegetable Research and Development Center, Shanhua, Taiwan, China
AVRDC (1980) AVRDC progress report 1980. Asian Vegetable Research and Development Center, Shanhua, Taiwan, China
AVRDC (1984) AVRDC progress report 1984. Asian Vegetable Research and Development Center, Shanhua, Taiwan, China
Bhattacharya S, Takada S, Jacobson RH (2007) Structural analysis and dimerization potential of the human TAF5 subunit of TFIID. Proc Natl Acad Sci USA 104:1189–1194. https://doi.org/10.1073/pnas.0610297104
Booker HM, Umaharan P (2008) Quantitative resistance to Cercospora leaf spot disease caused by Pseudocercospora cruenta in cowpea. Euphytica 162:167–177. https://doi.org/10.1007/s10681-007-9490-7
Burke TW, Kadonaga JT (1997) The downstream core promoter element, DPE, is conserved from Drosophila to humans and is recognized by TAFII60 of Drosophila. Genes Dev 11:3020–3031. https://doi.org/10.1101/gad.11.22.3020
Castro NR, Menezes GC, Coelho RSB (2003) Inheritance of cowpea resistance to Cercospora leaf spot. Fitopato Bras 28:552–554
Chalky GE, Verrijzer CP (1999) DNA binding site selection by RNA polymerase II TAFs: A TAF(II)250-TAF(II)150 complex recognizes the initiator. EMBO J 18:4835–4845. https://doi.org/10.1093/emboj/18.17.4835
Chankaew S, Somta P, Sorajjapinun W, Srinives P (2011) Quantitative trait loci mapping of Cercospora leaf spot resistance in mungbean, Vigna radiata (L.) Wilczek. Mol Breed 28:255–264. https://doi.org/10.1007/s11032-010-9478-1
Chotechung S, Somta P, Chen J, Yimram T, Chen X, Srinives P (2016) A gene encoding a polygalacturonase-inhibiting protein (PGIP) is a candidate gene for bruchid (Coleoptera: Bruchidae) resistance in mungbean (Vigna radiata). Theor Appl Genet 129:1673–1683. https://doi.org/10.1007/s00122-016-2731-1
Deighton FC (1976) Studies on Cercospora and allied genera.VI. Psuedocercospora Speg., Pantospora Cif. and Cercoseptoria Petr. Mycol Pap 140: 168
Dikstein R, Ruppert S, Tjian R (1996) TAFII250 is a bipartite protein kinase that phosphorylates the basal transcription factor RAP74. Cell 84:781–790. https://doi.org/10.1016/S0092-8674(00)81055-7
Dong OX, Meteignier LV, Plourde MB, Ahmed B, Wang M, Jensen C, Jin H, Moffett P, Li X, Germain H (2016) Arabidopsis TAF15b localizes to RNA processing bodies and contributes to snc1-mediated autoimmunity. Mol Plant-Microbe Interact 29:247–257. https://doi.org/10.1094/MPMI-11-15-0246-R
Duangsong U, Kaewwongwal A, Somta P, Chankaew S, Srinives P (2016) Identification of a major QTL for resistance to Cercospora leaf spot disease in cowpea (Vigna unguiculata (L.) Walp.) revealed common genomic region with that for the resistance to angular leaf spot in common bean (Phaseolus vulgaris L.). Euphytica 209:199–207. https://doi.org/10.1007/s10681-016-1662-x
Duangsong U, Laosatit K, Somta P, Srinives P (2018) Genetics of resistance to Cercospora leaf spot disease caused by Cercospora canescens and Pseudocercospora cruenta in yardlong bean (Vigna unguiculata ssp. sesquipedalis) × grain cowpea (V. unguiculata ssp. unguiculata) populations. J Genet 97:1451–1456. https://doi.org/10.1007/s12041-018-1003-z
Durso RJ, Fisher AK, Albright-Frey TJ, Reese JC (2001) Analysis of TAF90 mutants displaying allele-specific and broad defects in transcription. Mol Cell Biol 21:7331–7344. https://doi.org/10.1128/MCB.21.21.7331-7344.2001
Federer WT (1956) Augmented (or hoonuiaku) designs. Hawaiian planters’ record LV(2) pp 191–208
Fery RL, Dukes PD, Cuthbert FP Jr (1976) The inheritance of Cercospora leaf spot resistance in Southern pea (Vigna unguiculata (L.) Walp.). J Am Soc Hortic Sci 101:148–149
Grant PA, Schieltz D, Pray-Grant MG, Steger DJ, Reese JC, Yates J, Workman JL (1998) A subset of TAFIIs are integral components of the SAGA complex required for nucleosome acetylation and transcriptional stimulation. Cell 94:45–53. https://doi.org/10.1016/S0092-8674(00)81220-9
Grewal JS, Machendra P, Kulshrestha DP (1980) Control of Cercospora leaf spot of green gram by spraying Bavistin. Indian J Agric Sci 50:707–711
Gui CY, Ngo L, Xu WS, Richon VM, Marks PA (2004) Histone deacetylase (HDAC) inhibitor activation of p21WAF1 involves changes in promoter-associated proteins, including HDAC1. Proc Natl Acad Sci USA 101:1241–1246. https://doi.org/10.1073/pnas.0307708100
Hartl M, Fü M, Boersema PJ, Jost J, Kramer K, Bakirbas A et al (2017) Lysine acetylome profiling uncovers novel histone deacetylase substrate proteins in Arabidopsis. Mol Syst Biol 13:949. https://doi.org/10.15252/msb.20177819
Hartman GL, Wang TC, Kim D (1993) Field evaluation of mungbeans for resistance to Cercospora leaf spot and powdery mildew. Int J Pest Manag 39:418–421. https://doi.org/10.1080/09670879309371833
Heng T, Kaga A, Chen X, Somta P (2020) Two tightly linked genes coding for NAD-dependent malic enzyme and dynamin-related protein are associated with resistance to Cercospora leaf spot disease in cowpea (Vigna unguiculata (L.) Walp.). Theor Appl Genet 133:395–407. https://doi.org/10.1007/s00122-019-03470-6
Hoskin A (2011) Genetic mapping of soybean resistance genes to frogeye leaf spot in five Chinese plant introductions and efficiency of early generation selection for low phytate soybean lines. Institute of Plant Breeding, Genetics, and Genomics. Ph.D. Dissertation, University of Georgia, USA
Imhof A, Yang XJ, Ogryzko VV, Nakatani Y, Wolffe AP, Ge H (1997) Acetylation of general transcription factors by histone acetyltransferases. Curr Biol 7:689–692. https://doi.org/10.1016/S0960-9822(06)00296-X
Iqbal SM, Ghafoor A, Bashir M, Malik BA (1995) Estimation of losses in yield components of mugbean due to Cersospora leaf spot. Pak J Phytopathol 7:80–81
Kaewwongwal A, Chen J, Somta P, Kongjaimun A, Yimram T, Chen X, Srinives P (2017) Novel alleles of two tightly linked genes encoding polygalacturonase-inhibiting proteins (VrPGIP1 and VrPGIP2) associated with the Br locus that confer bruchid (Callosobruchus spp.) resistance to mungbean (Vigna radiata) accession V2709. Front Plant Sci 28:1692. https://doi.org/10.3389/fpls.2017.01692
Kaewwongwal A, Liu C, Somta P, Chen J, Tian J, Yuan X, Chen X (2019) A second VrPGIP1 allele is associated with bruchid resistance (Callosobruchus spp.) in wild mungbean (Vigna radiata var. sublobata) accession ACC41. Mol Genet Genom. https://doi.org/10.1007/s00438-019-01619-y
Kang YJ, Kim SK, Kim MY, Lestari P, Kim KH, Ha BK et al (2014) Genome sequence of mungbean and insights into evolution within Vigna species. Nat Commun 5:5443. https://doi.org/10.1038/ncomms6443
Kolesnikova O, Ben-Shem A, Luo J, Ranish J, Schultz P, Papai G (2018) Molecular structure of promoter-bound yeast TFIID. Nat Commun 9:4666. https://doi.org/10.1038/s41467-018-07096-y
Kouzarides T (2000) Acetylation: a regulatory modification to rival phosphorylation. EMBO J 19:1176–1179. https://doi.org/10.1093/emboj/19.6.1176
Laksana C, Chanprame S (2015) A simple and rapid method for RNA extraction from young and mature leaves of oil palm (Elaeis guineensis Jacq.). J ISSAAS 21:96–106
Leabwon U, Oupadissakoon S (1984) Inheritance of resistance to Cercospora leaf spot in mungbean. Kasetsart J (Nat Sci) 18:14–19
Lee YB (1980) Inheritance study on resistance to Cercospora leaf spot in mungbean. Asian Vegetable Research and Development Center, Shanhua, Taiwan, China
Leurent C, Sanders SL, Demény MA, Garbett KA, Ruhlmann C, Weil PA et al (2004) Mapping key functional sites within yeast TFIID. EMBO J 2:719–772. https://doi.org/10.1038/sj.emboj.7600111
Li H, Ye G, Wang J (2007) A modified algorithm for the improvement of composite interval mapping. Genetics 175:361–374. https://doi.org/10.1534/genetics.106.066811
Li S, Shi W, Leng RF, Leng Y (2015) De novo characterization of the mungbean transcriptome and transcriptomic analysis of adventitious rooting in seedlings using RNA-seq. PLoS ONE 10:e0132969. https://doi.org/10.1371/journal.pone.0132969
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔC. Methods 25:402–408. https://doi.org/10.1006/meth.2001.1262
Lodhi MA, Ye GN, Weeden NF, Reisch BI (1994) A simple and efficient method for DNA extraction from grapevine cultivars and Vitis species. Plant Mol Biol Rep 12:6–13. https://doi.org/10.1007/BF02668658
Lyer AS, McCouch SR (2004) The rice bacterial blight resistance gene xa5 encodes a novel form of disease resistance. Mol Plant-Microbe Interact 17:1348–1354. https://doi.org/10.1094/MPMI.2004.17.12.1348
Meng L, Li H, Zhang L, Wang J (2015) QTL IciMapping: integrated software for genetic linkage map construction and quantitative trait locus mapping in biparental populations. Crop J 3:269–283. https://doi.org/10.1016/j.cj.2015.01.001
Mian MAR, Wang T, Phillips DV, Alvernaz J, Boerma HR (1999) Molecular mapping of the Rcs3 gene for resistance to frogeye leaf spot of soybean. Crop Sci 39:1687–1691. https://doi.org/10.2135/cropsci1999.3961687x
Mishra SP, Asthana AN, Yadav L (1988) Inheritance of Cercospora leaf spot resistance in mungbean, Vigna radiata (L.) Wilczek. Plant Breed 100:228–229. https://doi.org/10.1111/j.1439-0523.1988.tb00245.x
Mizzen CA, Yang XJ, Kokubo T, Brownell JE, Bannister AJ, Owen-Hughes T et al (1996) The TAFII250 subunit of TFIID has histone acetyltransferase activity. Cell 87:1261–1270. https://doi.org/10.1016/S0092-8674(00)81821-8
Moraga F, Aquea F (2015) Composition of the SAGA complex in plants and its role in controlling gene expression in response to abiotic stresses. Front Plant Sci 6:865. https://doi.org/10.3389/fpls.2015.00865
Mougiou N, Poulios S, Kaldis A, Vlachonasios KE (2012) Arabidopsis thaliana TBP-associated factor 5 is essential for plant growth and development. Mol Breed 30:355–366. https://doi.org/10.1007/s11032-011-9626-2
Nair RM, Schafleitner R, Kenyon L, Srinivasan R, Easdown W, Ebert A et al (2012) Genetic improvement of mungbean. SABRAO J Breed Genet 44:177–190
Oelgeschlager T, Chiang CM, Roeder RG (1996) Topology and reorganization of a human TFIID-promoter complex. Nature 382:735–738. https://doi.org/10.1038/382735a0
Patel AB, Louder RK, Greber BJ, Grünberg S, Luo J, Fang J et al (2018) Structure of human TFIID and mechanism of TBP loading onto promoter DNA. Science 362:eaau8872
Pham A-T, Harris DK, Buck J, Hoskins A, Serrano J, Abdel-Haleem H, Cregan P, Song Q, Boerma HR, Li Z (2015) Fine mapping and characterization of candidate genes that control resistance to Cercospora sojina K. Hara in two soybean germplasm accessions. PLoS ONE 10(5):e0126753. https://doi.org/10.1371/journal.pone.0126753
Pookpakdi A, Promkham V, Chuangpetchinda C, Pongkao S, Lairungrueng C, Tawornsuk C (1992) Growth stage identification in mungbean (Vigna radiata (L.) Wilczek). Kasetsart J Nat Sci 26:75–80
Pray-Grant MG, Schieltz D, McMahon SJ, Wood JM, Kennedy EL, Cook RG et al (2002) The novel SLIK histone acetyltransferase complex functions in the yeast retrograde response pathway. Mol Cell Biol 22:8774–8786. https://doi.org/10.1128/MCB.22.24.8774-8786.2002
R Development Core Team (2010) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna
Rath GC, Grewal JS (1973) A note on Cercospora leaf spot of Phaseolus aureus. J Mycol Plant Pathol 3:204–207
Roeder RG (1996) The role of general initiation factors in transcription by RNA polymerase II. Trends Biochem Sci 21:327–335. https://doi.org/10.1016/S0968-0004(96)10050-5
Scheer E, Delbac F, Tora L, Moras D, Romier C (2012) TFIID TAF6-TAF9 complex formation involves the HEAT repeat-containing C-terminal domain of TAF6 and is modulated by TAF5 protein. J Biol Chem 287:27580–27592. https://doi.org/10.1074/jbc.M112.379206
Sievers F, Wilm A, Dineen DG, Gibson TJ, Karplus K, Li W et al (2011) Fast, scalable generation of high-quality protein multiple sequence alignments using Clustal Omega. Mol Syst Biol 7:539. https://doi.org/10.1038/msb.2011.75
Smith TF, Gaitatzes C, Saxena K, Neer EJ (1999) The WD40 repeat: a common architecture for diverse functions. Trends Biochem Sci 24:181–185. https://doi.org/10.1016/s0968-0004(99)01384-5
Tao Y, Guermah M, Martinez E, Oelgeschlager T, Hasegawa S, Takada R et al (1997) Specific interactions and potential functions of human TAFII100. J Biol Chem 272:6714–6721. https://doi.org/10.1074/jbc.272.10.6714
Temnykh S, DeClerck G, Lukashova A, Lipovich L, Cartinhour S, McCouch S (2001) Computational and experimental analysis of microsatellites in rice (Oryza sativa L.): frequency, length variation, transposon associations, and genetic marker potential. Genome Res 11:1441–1452. https://doi.org/10.1101/gr.184001
Thakur RP, Patel PN, Verma JP (1977a) Genetical relationships between reactions to bacterial leaf spot, yellow mosaic and Cercopsora leaf spot diseases in mungbean (Vigna radiata). Euphytica 26:765–774. https://doi.org/10.1007/BF00021705
Thakur RP, Patel PN, Verma JP (1977b) Independent assortment of pigmentation and resistance to Cercospora leaf spot diseases in mungbean. Indian Phytopathol 30:264–265
Tickoo JL, Satyanarayana A (1998) Progress in mungbean breeding research with special emphasis on disease and insect resistance, constraints and future directions. In: Shanmugasundaram S (ed) Proceedings of the international consultation workshop on mungbean. Shanhua, Taiwan, China
Tora L (2002) A unified nomenclature for TATA box binding protein (TBP)-associated factors (TAFs) involved in RNA polymerase II transcription. Genes Dev 16:673–675
Untergasser A, Cutcutache I, Koressaar T, Ye J, Faircloth BC, Remm M, Rozen SG (2012) Primer3—new capabilities and interfaces. Nucleic Acids Res 40:e115. https://doi.org/10.1093/nar/gks596
Verrijzer CP, Yokomori K, Chen JL, Tjian R (1994) Drosophila TAFII150: similarity to yeast gene TSM-1 and specific binding to core promoter DNA. Science 264:933–941. https://doi.org/10.1126/science.8178153
Verrijzer CP, Chen JL, Yokomori K, Tjian R (1995) Binding of TAFs to core elements directs promoter selectivity by RNA polymerase II. Cell 87:1115–1125. https://doi.org/10.1016/S0092-8674(05)80016-9
Walley JW, Shen Z, McReynolds MR, Schmelz EA, Briggs SP (2018) Fungal-induced protein hyperacetylation in maize identified by acetylome profiling. Proc Natl Acad Sci USA 115:210–215. https://doi.org/10.1073/pnas.1717519115
Wright KJ, Marr MT, Tjian R (2006) TAF4 nucleates a core subcomplex of TFIID and mediates activated transcription from a TATA-less promoter. Proc Natl Acad Sci USA 103:12347–12352. https://doi.org/10.1073/pnas.0605499103
Zhang H, GaoZ Zheng X, Zhang Z (2012) The role of G-proteins in plant immunity. Plant Signal Behav 7:1284–1288. https://doi.org/10.4161/psb.21431
Acknowledgements
We are thankful to the Joint Legume Research Center between Jiangsu Academy of Agriculture Sciences and Kasetsart University for molecular lab facilities.
Funding
This work was supported by the National Key Research and Development Program of China (Grant No. 2016YFE0203800), the China Agricultural Research System (CARS-08), and the Jiangsu Agriculture Industry Technology System (Grant No. JATS[2018]255).
Author information
Authors and Affiliations
Contributions
PS conceived and designed the study. CY conducted all of the experiments. JC and XY participated in marker, DNA sequencing, and gene expression analyses. CY, PS, and SC developed the populations and conducted phenotyping. XC and PS acquired funding. PS and CX supervised the study. CY, PS, and XC wrote the manuscript. PS edited and revised the manuscript. All authors reviewed the manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
On behalf of all authors, the corresponding authors declare that they have no conflict of interest.
Ethical statement
The authors declare that this research has no human and animal participants and that the experiments comply with the current laws of the country in which they were carried out.
Additional information
Communicated by Henry T. Nguyen.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary file 1 (PDF 310 kb)
Supplementary Table S1 PCR primers used in amplification of a genomic region covering LOC106765332. Annealing temperature for all primers is 55-60 °C
Supplementary file 2 (PDF 563 kb)
Supplementary Table S2 PCR primers designed for real-time PCR analysis of LOC106765332 and the internal reference genes. Annealing temperature for all primers is 55°C
Supplementary file 3 (PDF 409 kb)
Supplementary Table S3 Mungbean SSR primers covering the marker intervals for qCLS developed in this study. Annealing temperature for all primers is 55°C
Supplementary file 4 (PDF 88 kb)
Supplementary Fig. S1 Scheme showing procedure for the development of BC8F2 mungbean population used for QTL mapping of Cercospora leaf spot resistance
Supplementary file 5 (PDF 190 kb)
Supplementary Fig. S2 Full sequence alignment of VrTAF5 (LOC106765332) from KPS1, V4718, and VC1973A (reference sequence)
Supplementary file 6 (PDF 178 kb)
Supplementary Fig. S3 Sequence alignment of the coding sequence of VrTAF5 (LOC106765332) from KPS1, V4718, and VC1973A (reference sequence)
Rights and permissions
About this article
Cite this article
Yundaeng, C., Somta, P., Chen, J. et al. Fine mapping of QTL conferring Cercospora leaf spot disease resistance in mungbean revealed TAF5 as candidate gene for the resistance. Theor Appl Genet 134, 701–714 (2021). https://doi.org/10.1007/s00122-020-03724-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00122-020-03724-8