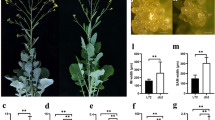
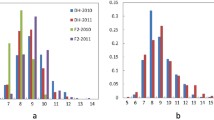
Abstract
Key message
A candidate branching-controlling gene for qDBA09 was identified after delimiting a Brassica napus recessive locus within a 270-kb interval on chromosome A09.
Abstract
Although branching is an important trait associated with the adaptation and yield potential of rapeseed (Brassica napus), the genetic mechanisms underlining branching in this crop remain poorly understood. In this study, we characterized a naturally occurring rapeseed mutant, db1, which showed an ultrahigh branching density phenotype. By combining bulked segregant analysis (BSA) and the Brassica 60K SNP BeadChip Array, we identified two major quantitative trait loci (QTLs), qDBA09 and qDBC06, which were subsequently confirmed using the traditional QTL-mapping approach. Analysis of 208 individuals from a BC1F3 population indicated that the qDBA09 locus is a single Mendelian factor and that the dense branching phenotype is controlled by a single recessive gene. Furthermore, QTL analysis confirmed that qDBA09 explained between 9.5 and 70.5% of the variation in branching-related traits. Using 7785 individuals from the BC1F3 population, we mapped qDBA09 to a DNA fragment of approximately 270 kb in length that contained 27 predicted genes, three of which were identified as potentially involved in the control of the dense branching trait. Based on the reported function of these genes, together with sequence comparisons and co-segregation analysis, we identified a potential candidate gene for the qDBA09 locus. The present findings lay the foundations for further in-depth research on the branching mechanisms of B. napus.







Similar content being viewed by others
References
Benjamins R, Quint A, Weijers D, Hooykaas P, Offringa R (2001) The PINOID protein kinase regulates organ development in Arabidopsis by enhancing polar auxin transport. Development 128:4057–4067
Cai G, Yang Q, Chen H, Yang Q, Zhang C, Fan C, Zhou Y (2016) Genetic dissection of plant architecture and yield-related traits in Brassica napus. Sci Rep 6:21625
Chalhoub B, Denoeud F, Liu S, Parkin IA, Tang H, Wang X, Chiquet J, Belcram H, Tong C, Samans B, Correa M, Da Silva C, Just J, Falentin C, Koh CS, Le Clainche I, Bernard M, Bento P, Noel B, Labadie K, Alberti A, Charles M, Arnaud D, Guo H, Daviaud C, Alamery S, Jabbari K, Zhao M, Edger PP, Chelaifa H, Tack D, Lassalle G, Mestiri I, Schnel N, Le Paslier MC, Fan G, Renault V, Bayer PE, Golicz AA, Manoli S, Lee TH, Thi VH, Chalabi S, Hu Q, Fan C, Tollenaere R, Lu Y, Battail C, Shen J, Sidebottom CH, Wang X, Canaguier A, Chauveau A, Berard A, Deniot G, Guan M, Liu Z, Sun F, Lim YP, Lyons E, Town CD, Bancroft I, Wang X, Meng J, Ma J, Pires JC, King GJ, Brunel D, Delourme R, Renard M, Aury JM, Adams KL, Batley J, Snowdon RJ, Tost J, Edwards D, Zhou Y, Hua W, Sharpe AG, Paterson AH, Guan C, Wincker P (2014) Early allopolyploid evolution in the post-Neolithic Brassica napus oilseed genome. Science 345:950–953
Chen W, Zhang Y, Liu X, Chen B, Tu J, Tingdong F (2007) Detection of QTL for six yield-related traits in oilseed rape (Brassica napus) using DH and immortalized F2 populations. Theor Appl Genet 115:849–858
Chen B, Xu K, Li J, Li F, Qiao J, Li H, Gao G, Yan G, Wu X (2014) Evaluation of yield and agronomic traits and their genetic variation in 488 global collections of Brassica napus L. Genet Resour Crop Evol 61:979–999
Christensen SK, Dagenais N, Chory J, Weigel D (2000) Regulation of auxin response by the protein kinase PINOID. Cell 100:469–478
Clarke JM, Simpson GM (1978) Influence of irrigation and seeding rates on yield and yield components of Brassica napus cv. tower. Can J Plant Sci 58:731–737
Clarke WE, Higgins EE, Plieske J, Wieseke R, Sidebottom C, Khedikar Y, Batley J, Edwards D, Meng J, Li R, Lawley CT, Pauquet J, Laga B, Cheung W, Iniguez-Luy F, Dyrszka E, Rae S, Stich B, Snowdon RJ, Sharpe AG, Ganal MW, Parkin IA (2016) A high-density SNP genotyping array for Brassica napus and its ancestral diploid species based on optimised selection of single-locus markers in the allotetraploid genome. Theor Appl Genet 129:1887–1899
Doyle JJ, Doyle JL (1990) Isolation of plant DNA from fresh tissue. Focus 12:13–15
Friml J, Yang X, Michniewicz M, Weijers D, Quint A, Tietz O, Benjamins R, Ouwerkerk PB, Ljung K, Sandberg G, Hooykaas PJ, Palme K, Offringa R (2004) A PINOID-dependent binary switch in apical-basal PIN polar targeting directs auxin efflux. Science 306:862–865
Fu TD, Yang GS, Tu JX, Ma CZ (2003) The present and future of rapeseed production in China. China Oils Fats 28:11–13
Giovannoni JJ, Wing RA, Ganal MW, Tanksley SD (1991) Isolation of molecular markers from specific chromosomal intervals using DNA pools from existing mapping populations. Nucleic Acids Res 19:6553–6568
Hu L, Zhang H, Yang Q, Meng Q, Han S, Nwafor CC, Khan MHU, Fan C, Zhou Y (2018) Promoter variations in a homeobox gene, BnA10.LMI1, determine lobed leaves in rapeseed (Brassica napus L.). Theor Appl Genet 131:2699–2708
Knauer S, Holt AL, Rubio-Somoza I, Tucker EJ, Hinze A, Pisch M, Javelle M, Timmermans MC, Tucker MR, Laux T (2013) A protodermal miR394 signal defines a region of stem cell competence in the Arabidopsis shoot meristem. Dev Cell 24:125–132
Kosambi DD (1944) The estimation of map distances from recombination values. Ann Eugen 12:172–175
Li Y, Takagi Y, Jiang Y, Tokunaga M, Erdjument-Bromage H, Tempst P, Kornberg RD (2001) A multiprotein complex that interacts with RNA polymerase II elongator. J Biol Chem 276:29628–29631
Li Y, Shen J, Wang T, Chen Q, Zhang X, Fu T, Meng J, Tu J, Ma C (2007) QTL analysis of yield-related traits and their association with functional markers in Brassica napus L. Crop Pasture Sci 58:759–766
Li F, Chen B, Xu K, Gao G, Yan G, Qiao J, Li J, Li H, Li L, Xiao X, Zhang T, Nishio T, Wu X (2016a) A genome-wide association study of plant height and primary branch number in rapeseed (Brassica napus). Plant Sci 242:169–177
Li H, Zhu L, Yuan G, Heng S, Yi B, Ma C, Shen J, Tu J, Fu T, Wen J (2016b) Fine mapping and candidate gene analysis of an anthocyanin-rich gene, BnaA.PL1, conferring purple leaves in Brassica napus L. Mol Genet Genom 291:1523–1534
Li X, Wang A, Zu F, Hu Z, Lin J, Zhou G, Tu J (2016c) Identification of a nuclear-recessive gene locus for male sterility on A2 chromosome using the Brassica 60 K SNP array in non-heading Chinese cabbage. Genes Genom 38:1151–1157
Lincoln S, Daly M, Lander ES (1992a) Constructing genetics maps with MAPMAKER/EXP 3.0. Whitehead Institute technical report, 3rd edn. Whitehead Institute, Cambridge
Lincoln S, Daly M, Lander ES (1992b) Mapping genes controlling quantitative traits with MAPMAKER/QTL 1.1. Whitehead Institute technical report, 2nd edn. Massachusetts, Whitehead Institute
MacAlister CA, Park SJ, Jiang K, Marcel F, Bendahmane A, Izkovich Y, Eshed Y, Lippman ZB (2012) Synchronization of the flowering transition by the tomato TERMINATING FLOWER gene. Nat Genet 44:1393–1398
Mallory AC, Hinze A, Tucker MR, Bouche N, Gasciolli V, Elmayan T, Lauressergues D, Jauvion V, Vaucheret H, Laux T (2009) Redundant and specific roles of the ARGONAUTE proteins AGO1 and ZLL in development and small RNA-directed gene silencing. PLoS Genet 5:e1000646
McSteen P, Leyser O (2005) Shoot branching. Annu Rev Plant Biol 56:353–374
Michelmore RW, Paran I, Kesseli RV (1991) Identification of markers linked to disease-resistance genes by bulked segregant analysis: a rapid method to detect markers in specific genomic regions by using segregating populations. Proc Natl Acad Sci U S A 88:9828–9832
Nelissen H, Fleury D, Bruno L, Robles P, De Veylder L, Traas J, Micol JL, Van Montagu M, Inze D, Van Lijsebettens M (2005) The elongata mutants identify a functional Elongator complex in plants with a role in cell proliferation during organ growth. Proc Natl Acad Sci U S A 102:7754–7759
Nelissen H, De Groeve S, Fleury D, Neyt P, Bruno L, Bitonti MB, Vandenbussche F, Van der Straeten D, Yamaguchi T, Tsukaya H, Witters E, De Jaeger G, Houben A, Van Lijsebettens M (2010) Plant Elongator regulates auxin-related genes during RNA polymerase II transcription elongation. Proc Natl Acad Sci U S A 107:1678–1683
Petrasek J, Mravec J, Bouchard R, Blakeslee JJ, Abas M, Seifertova D, Wisniewska J, Tadele Z, Kubes M, Covanova M, Dhonukshe P, Skupa P, Benkova E, Perry L, Krecek P, Lee OR, Fink GR, Geisler M, Murphy AS, Luschnig C, Zazimalova E, Friml J (2006) PIN proteins perform a rate-limiting function in cellular auxin efflux. Science 312:914–918
Qiu D, Morgan C, Shi J, Long Y, Liu J, Li R, Zhuang X, Wang Y, Tan X, Dietrich E, Weihmann T, Everett C, Vanstraelen S, Beckett P, Fraser F, Trick M, Barnes S, Wilmer J, Schmidt R, Li J, Li D, Meng J, Bancroft I (2006) A comparative linkage map of oilseed rape and its use for QTL analysis of seed oil and erucic acid content. Theor Appl Genet 114:67–80
Salvi S, Tuberosa R (2005) To clone or not to clone plant QTLs: present and future challenges. Trends Plant Sci 10:297–304
Shen W, Qin P, Yan M, Li B, Wu Z, Wen J, Yi B, Ma C, Shen J, Fu T, Tu J (2019) Fine mapping of a silique length- and seed weight-related gene in Brassica napus. Theor Appl Genet 132:2985–2996
Shi J, Li R, Qiu D, Jiang C, Long Y, Morgan C, Bancroft I, Zhao J, Meng J (2009) Unraveling the complex trait of crop yield with quantitative trait loci mapping in Brassica napus. Genetics 182:851–861
Somssich M, Je BI, Simon R, Jackson D (2016) CLAVATA-WUSCHEL signaling in the shoot meristem. Development 143:3238–3248
Tuncturk M, Çiftçi V (2007) Relationships between yield and some yield components in rapeseed (Brassica napus ssp. oleifera L.) cultivars by using correlation and path analysis. Pak J Bot 39:81–84
Versées W, De Groeve S, Van Lijsebettens M (2010) Elongator, a conserved multitasking complex? Mol Microbiol 76:1065–1069
Wang Y, Jiao Y (2018) Axillary meristem initiation-a way to branch out. Curr Opin Plant Biol 41:61–66
Wang Y, He J, Yang L, Wang Y, Chen W, Wan S, Chu P, Guan R (2016) Fine mapping of a major locus controlling plant height using a high-density single-nucleotide polymorphism map in Brassica napus. Theor Appl Genet 129:1479–1491
Wang B, Smith SM, Li J (2018) Genetic regulation of shoot architecture. Annu Rev Plant Biol 69:437–468
Winkler GS, Kristjuhan A, Erdjument-Bromage H, Tempst P, Svejstrup JQ (2002) Elongator is a histone H3 and H4 acetyltransferase important for normal histone acetylation levels in vivo. Proc Natl Acad Sci U S A 99:3517–3522
Xiao L, Yi B, Chen Y, Huang Z, Chen W, Ma C, Tu J, Fu T (2008) Molecular markers linked to Bn;rf: a recessive epistatic inhibitor gene of recessive genic male sterility in Brassica napus L. Euphytica 164:377–384
Xu J, Song X, Cheng Y, Zou X, Zeng L, Qiao X, Lu G, Fu G, Qu Z, Zhang X (2014) Identification of QTLs for branch number in oilseed rape (Brassica napus L.). J Genet Genom 41:557–559
Zhao X, Li B, Zhang K, Hu K, Yi B, Wen J, Ma C, Shen J, Fu T, Tu J (2016) Breeding signature of combining ability improvement revealed by a genomic variation map from recurrent selection population in Brassica napus. Sci Rep 6:29553
Zheng M, Peng C, Liu H, Tang M, Yang H, Li X, Liu J, Sun X, Wang X, Xu J, Hua W, Wang H (2017) Genome-wide association study reveals candidate genes for control of plant height, branch initiation height and branch number in rapeseed (Brassica napus L.). Front Plant Sci 8:1246
Acknowledgments
This work was financed by the National Key Research and Development Program of China (Grant Number 2016YFD0100305).
Author information
Authors and Affiliations
Contributions
BL performed the experiments and prepared the manuscript. JG and JC helped with the experiments. ZW and WS helped analyze the data. JW, BY, CM, and TF gave advises to the experimental design. JT conceived the project and revised the manuscript.
Corresponding author
Ethics declarations
Ethical standards
The authors declare that this study complies with current laws of China.
Conflict of interest
All authors declare that they have no conflict of interest.
Additional information
Communicated by Maria Laura Federico.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Fig. S1
. Additional branching phenotypes associated the db1 alleles. Fasciated inflorescence of db1 (a). Primary inflorescence of T109 (b) and db1 (c). Bar = 5 cm (TIF 15944 kb)
Supplementary Fig. S2
. Alignment of the amino acid sequences of candidate genes that are polymorphic between T109 and db1. (TIF 11602 kb)
Supplementary Fig. S3
. Relative expressions of the candidate genes that exhibited a higher expression in the SAM of TT plants. Asterisks indicate significant differences between plants homozygous for db1 (DD) and T109 (TT). (TIF 947 kb)
Supplementary Fig. S4
. Alignment of the gDNA sequences of db1 and T109. BnaA09.ELP6, T109, and db1 denote the sequences of Darmor-bzh, T109, and db1, respectively. (TIF 20100 kb)
Supplementary Table S1
. Molecular markers developed in this study. IP, intron polymorphism; SSR, simple sequence repeat. (XLSX 10 kb)
Supplementary Table S2
. Primers designed for gene cloning, vector construction and expression analysis. (XLSX 13 kb)
Supplementary Table S3
. Single-nucleotide polymorphisms between “dense branching” bulks and “normal branching” bulks. (XLSX 33 kb)
Supplementary Table S4
. Co-segregation analysis using 176 recombinants from the BC1F3 population. aPhenotype of the plants: DD, dense branching; TT, normal branching; INTER, intermediate. bGenotype of maker P12: DD, homozygous for the db1 allele; TT, homozygous for the T109 allele; and H, heterozygous. cThree classes were identified for the qDBA09 genotype [homozygous for db1 (DD) and T109 (TT) and heterozygote (H)] using a progeny test. (XLSX 50 kb)
Supplementary Table S5
. Predicted Brassica napus genes in the candidate region of the qDBA09 locus, and the best hits for these genes among Arabidopsis thaliana proteins using BLAST. (XLSX 11 kb)
Supplementary Table S6
. Co-segregation analysis using 208 plants from the BC1F3 population. Three classes were identified for the qDBA09 genotype [homozygous for db1 (DD) and T109 (TT) and heterozygote (H)] using a progeny test. (XLSX 16 kb)
Rights and permissions
About this article
Cite this article
Li, B., Gao, J., Chen, J. et al. Identification and fine mapping of a major locus controlling branching in Brassica napus. Theor Appl Genet 133, 771–783 (2020). https://doi.org/10.1007/s00122-019-03506-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00122-019-03506-x