Abstract
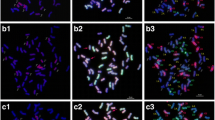
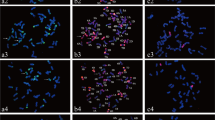
In situ hybridization (multicolor GISH and FISH) was used to characterize the genomic composition of the wheat–Thinopyrum ponticum partial amphiploid BE-1. The amphiploid is a high-protein line having resistance to leaf rust (Puccinia recondita f. sp. tritici) and powdery mildew (Blumeria graminis f. sp. tritici) and has in total 56 chromosomes per cell. Multicolor GISH using J, A and D genomic probes showed 16 chromosomes originating from Thinopyrum ponticum and 14 A genome, 14 B genome and 12 D genome chromosomes. Six of the Th. ponticum chromosomes carried segments different from the J genome in their centromeric regions. It was demonstrated that these alien chromosome segments did not originate from the A, B or D genomes of wheat, so the translocation chromosomes were considered to be Js type chromosomes carrying segments similar to the S genome near the centromeres. Rearrangements between the A and D genomes of wheat were detected. FISH using Afa family, pSc119.2 and pTa71 probes allowed the identification of all the wheat chromosomes present and the determination of the chromosomes involved in the translocations. The 4A and 7A chromosomes were identified as being involved in intergenomic translocations. The replaced wheat chromosome was identified as 7D. The localization of these repetitive DNA clones on the Th. ponticum chromosomes of the amphiploid was described in the present study. On the basis of their multicolor FISH patterns, the alien chromosomes could be arranged in eight pairs and could also be differentiated unequivocally from each other.



Similar content being viewed by others
References
Anderson JA, Ogihara Y, Sorrells ME, Tanksley SD (1992) Development of chromosomal arm map for wheat based on RFLP markers. Theor Appl Genet 83:1035–1043
Bedbrook J, Jones J, O’Dell M, Thompson RD, Flavell RB (1980) A molecular description of telomeric heterochromatin in Secale species. Cell 19:545–560
Bommineni VR, Jauhar PP (1997) Wide hybridization and genome relationships in cereals: an assessment of molecular approaches. Maydica 42:81–105
Brasileiro-Vidal A, Cuadrado A, Brammer SP, Zanatta ACA, Prestes AM, Moraes-Fernandes MIB, Guerra M (2003) Chromosome characterisation in Thinopyrum ponticum (Triticeae, Poaceae) using in situ hybridization with different DNA sequences. Genet Mol Biol 26:505–510
Cai X, Jones S (1997) Direct evidence for high level of autosyndetic pairing in hybrids of Thinopyrum intermedium and Th. ponticum with Triticum aestivum. Theor Appl Genet 95:568–572
Cai X, Jones SS, Murray TD (2001) Molecular cytogenetic characterization of Thinopyrum genomes conferring perennial growth habit in wheat–Thinopyrum amphiploids. Plant Breed 120:21–26
Cauderon Y (1966) Genome analysis in the genus Agropyron. Proceedings of the 2nd International Wheat Genetics Symposium. Hereditas 2(Suppl.):218–234
Chao S, Sharp PJ, Worland AJ, Warham EJ, Koebner RMD, Gale MD (1989) RFLP-based genetic maps of wheat homoeologous group 7 chromosomes. Theor Appl Genet 78:495–504
Chen Q, Ahmad F, Collin J, Comeau A, Fedak G, St-Pierre CA (1998a) Genomic constitution of a partial amphiploid OK7211542 used as a source of immunity to barley yellow dwarf virus for bread wheat. Plant Breed 117:1–6
Chen Q, Conner RL, Ahmad F, Laroche A, Fedak G, Thomas JB (1998b) Molecular characterization of the genome composition of partial amphiploids derived from Triticum aestivum × Thinopyrum ponticum and T. aestivum × Th. intermedium as sources of resistance to wheat streak mosaic virus and its vector, Aceriella tosichella. Theor Appl Genet 97:1–8
Chen Q, Conner RL, Laroche A, Thomas JB (1998c) Genome analysis of Thinopyrum intermedium and Thinopyrum ponticum using genomic in situ hybridization. Genome 41:580–586
Contento A, Heslop-Harrison JS, Schwarzacher T (2005) Diversity of a major repetitive DNA sequence in diploid and polyploid Triticeae. Cytogenet Genome Res 109:34–42
Devos KM, Dubcovsky J, Dvorák J, Chinoy CN (1995) Structural evolution of wheat chromosomes 4A, 5A, and its impact on recombination. Theor Appl Genet 91:282–288
Dvorak J (1981) Genome relationships among Elytrigia (= Agropyron) elongata, E. stipifolia, ‘E. elongata 4x’, E. caespitosa, E. intermedia, and ‘E. elongata 10x’. Can J Genet Cytol 23:481–492
Ellneskog-Staam P, Merker A (2002) Chromosome composition, stability and fertility of alloploids between Triticum turgidum var. carthlicum and Thinopyrum junceiforme. Hereditas 136:59–65
Fedak G, Han F (2005) Characterization of derivatives from wheat–Thinopyrum wide crosses. Cytogenet Genome Res 109:360–367
Fedak G, Chen Q, Conner RL, Laroche A, Petroski R, Armstrong KW (2000) Characterisation of wheat–Thinopyrum partial amphiploids by meiotic analysis and genomic in situ hybridization. Genome 43:712–719
Friebe B, Jiang J, Raupp WJ, McIntosh RA, Gill BS (1996) Characterisation of wheat-alien translocations conferring resistance to diseases and pests: current status. Euphytica 91:59–87
Gale MD, Miller TE (1987) The introduction of alien genetic variation in wheat. In: Lupton FGH (ed) Wheat breeding: its scientific basis. Chapman and Hall, London, pp 173–177
Gerlach WL, Bedbrook JR (1979) Cloning and characterization of ribosomal RNA genes from wheat and barley. Nucleic Acids Res 7:1869–1885
Han FP, Fedak G, Benabdelmouna A, Armstrong K, Ouellet T (2003) Characterization of six wheat–Thinopyrum intermedium derivatives by GISH, RFLP, and multicolor GISH. Genome 46:490–495
Han F, Liu B, Fedak G Liu Z (2004) Genomic constitution and variation in five partial amphiploids of wheat–Thinopyrum intermedium as revealed by GISH, multicolor GISH and seed storage protein analysis. Theor Appl Genet 109:1070–1076
Jauhar PP (1995) Meiosis and fertility of F1 hybrids between hexaploid bread wheat and decaploid tall wheatgrass (Thinopyrum ponticum). Theor Appl Genet 90:865–871
Jiang J, Friebe B, Dhaliwal HS, Martin TJ, Gill BS (1993) Molecular cytogenetic analysis of Agropyron elongatum chromatin in wheat germplasm specifying resistance to wheat streak mosaic virus. Theor Appl Genet 86:41–48
Jiang J, Friebe B, Gill BS (1994) Recent advances in alien gene transfer in wheat. Euphytica 73:199–212
Knott DR (1968) Translocations involving Triticum chromosomes carrying rust resistance. Can J Genet Cytol 10:695–696
Knott DR (1987) Transferring alien genes to wheat. In: Heyne EG (ed) Wheat and wheat improvement, 2nd edn, Monogr 13. American Society of Agronomics, pp 462–471
Kubaláková M, Kovárová P, Suchánková P, Cíhalíková J, Bartos J, Lucretti S, Watanabe N, Kianian SF, Dolezel J (2005) Chromosome sorting in tetraploid wheat and its potential for genome analysis. Genetics 170:823–829
Le HT, Armstrong KC, Miki B (1989) Detection of rye DNA in wheat–rye hybrids and wheat translocation stocks using total genomic DNA as a probe. Plant Mol Biol Rep 7:150–158
Li H, Conner RL, Chen Q, Li H, Laroche A, Graf RJ, Kuzyk AD (2004) The transfer and characterization of resistance to common root rot from Thinopyrum ponticum to wheat. Genome 47:215–223
Li H, Chen Q, Conner RL, Guo B, Zhang Y, Graf RJ, Laroche A, Jia X, Liu G, Chu C (2003) Molecular characterization of a wheat–Thinopyrum ponticum partial amphiploid and its derivatives for resistance to leaf rust. Genome 46:906–913
Li D, Zhang X (2002) Physical localisation of the 18S-5.8S-25S rDNA and sequence analysis of ITS regions in Thinopyrum ponticum (Poaceae: Triticeae): implications for concerted evolution. Ann Bot 90:445–452
Linc G, Friebe B, Kynast RG, Molnár-Láng M, Kőszegi B, Sutka J, Gill BS (1999) Molecular cytogenetic analysis of Aegilops cylindrica Host. Genome 42:497–503
Liu CJ, Atkinson MD, Chinoy CN, Devos KM, Gale MD (1992) Nonhomoeologous translocations between group 4, 5 and 7 chromosomes within wheat and rye. Theor Appl Genet 83:305–312
Lukaszewski AJ, Rybka K, Korzun V, Malyshev SV, Lapinski B, Whitkus R (2004) Genetic and physical mapping of homoeologous recombination points involving wheat chromosome 2B and rye chromosome 2R. Genome 47:36–45
Martin TJ, Harvey TL, Livers RW (1976) Resistance to wheat streak mosaic virus and its vector, Aceria tulipae. Phytopathology 66:346–349
Molnár-Láng M, Linc G, Friebe R B, Sutka J (2000) Detection of wheat–barley translocations by genomic in situ hybridization in derivatives of hybrids multiplied in vitro. Euphytica 112:117–123
Mukai Y, Nakahara Y, Yamamoto M (1993) Simultaneous discrimination of three genomes in hexaploid wheat by multicolor fluorescence in situ hybridization using total genomic and highly repeated DNA probes. Genome 36:489–494
Muramatsu M (1990) Cytogenetics of decaploid Agropyron elongatum (Elytrigia elongata) (2n = 70). I. Frequency of decavalent formation. Genome 33:811–817
Nagaki K, Tsujimoto H, Isono K, Sasakuma T (1995) Molecular characterization of a tandem repeat, Afa family, and its distribution among Triticeae. Genome 38:479–486
Nagy ED, Molnár-Láng M, Linc G, Láng L (2002) Identification of wheat–barley translocations by sequential GISH and two-colour FISH in combination with the use of genetically mapped barley SSR markers. Genome 45:1238–1247
Naranjo T, Roca P, Goicoechea PG, Giraldez R (1987) Arm homoeology of wheat and rye chromosomes. Genome 29:873–882
Naranjo T (1990) Chromosome structure of durum wheat. Theor Appl Genet 79:397–400
Oliver RE, Xu SS, Stack RW, Friesen TL, Jin Y, Cai X (2006) Molecular cytogenetic characterization of four partial wheat–Thinopyrum ponticum amphiploids and their reaction to Fusarium head blight, tan spot, and Stagonospora nodorum blotch. Theor Appl Genet 112:1473–1479
Pedersen C, Langridge P (1997) Identification of entire chromosome complement of bread wheat by two-colour FISH. Genome 40:589–593
Pestsova E, Ganal MW, Röder MS (2000) Isolation and mapping of microsatellite markers specific for the D genome of bread wheat. Genome 43:689–697
Rayburn AL, Gill BS (1986) Molecular identification of the D-genome chromosomes of wheat. J Hered 77:253–255
Rayburn AL, Gill BS (1987) Isolation of D-genome specific repeated DNA sequence from Aegilops squarrosa. Plant Mol Biol Rep 4:102–109
Röder M, Korzun V, Wendehake K, Plaschke J, Tixier MH, Leroy P, Ganal MW (1998a) A microsatellite map of wheat. Genetics 149:2007–2023
Röder M, Korzun V, Gill BS, Ganal MW (1998b) The physical mapping of microsatellite markers in wheat. Genome 41:278–283
Schwarzacher T, Leitch AR, Bennett MD, Heslop-Harrison JS (1989) In situ localization of parental genomes in a wide hybrid. Ann Bot 64:315–324
Sánchez-Morán E., Benavente E., Orellana J (1998) Simultaneous identification of A, B, D and R genomes by genomic in situ hybridization in wheat–rye derivatives. Heredity 83:249–252
Sears ER (1973) Agropyron–wheat transfers induced by homoeologous pairing. Proceedings of the 4th International Wheat Genetics Symposium, Columbia, MO, USA, August 6–11, pp 191–199
Sharma D, Knott DR (1966) The transfer of leaf-rust resistance from Agropyron to Triticum by irradiation. Can J Genet Cytol 8:137–143
Sharma HC, Ohm HW, Perry KL (1997) Registration of barley yellow dwarf virus-resistant wheat germplasm line P29. Crop Sci 37:1032–1033
Sharp PJ, Kreis M, Shewry PR, Gale D (1988) Location of beta-amylase sequences in wheat and its relatives. Theor Appl Genet 75:286–290
Somers DJ, Isaac P, Edwards K (2004) A high-density microsatellite consensus map for bread wheat. Theor Appl Genet 109:1105–1114
Szalay D (1979) Faj- és nemzetséghibridek felhasználása a búzanemesítésben (Use of interspecific and intergenomic hybrids in wheat breeding). In: Bálint A (ed) A búza jelene és jövője (The present and future of wheat). Mezőgazdasági Kiadó, Budapest, pp 61–66
Thomas J, Chen Q, Talbert L (1998) Genetic segregation and the detection of spontaneous wheat-alien translocations. Euphytica 100:261–267
Vrana J, Kubaláková M, Simková H, Cihaliková J, Lysák MA, Dolezel J (2000) Flow sorting of mitotic chromosomes in common wheat (Triticum aestivum L.). Genetics 156:2033–2041
Wang J, Xiang F, Xia G (2005) Agropyron elongatum chromatin localization on the wheat chromosomes in an introgression line. Planta 221:277–286
Zhang P, Li W, Friebe B, Gill SB (2004) Simultaneous painting of three genomes in hexaploid wheat by BAC-FISH. Genome 47:979–987
Acknowledgments
This study was supported by a Hungarian GVOP grant (GVOP-3.1.1.-2004-05-0007/3.0) and by the Hungarian Wheat Ear Consortium (OM-00018/2004). The authors gratefully acknowledge the technical assistance of Mrs. I. Bucsi and Mrs. J. Havasi. Thanks are due to Dr. G. Linc and Dr. E. Benavente for their useful comments on the results and to B. Harasztos for revising the manuscript linguistically.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by B. Friebe.
Rights and permissions
About this article
Cite this article
Sepsi, A., Molnár, I., Szalay, D. et al. Characterization of a leaf rust-resistant wheat–Thinopyrum ponticum partial amphiploid BE-1, using sequential multicolor GISH and FISH. Theor Appl Genet 116, 825–834 (2008). https://doi.org/10.1007/s00122-008-0716-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00122-008-0716-4