Abstract
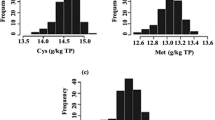
Soybean [Glycine max (L.) Merr.] is the single largest source of protein in animal feed. However, a major limitation of soy proteins is their deficiency in sulfur-containing amino acids, methionine (Met) and cysteine (Cys). The objective of this study was to identify quantitative trait loci (QTL) associated with Met and Cys concentration in soybean seed. To achieve this objective, 101 F6-derived recombinant inbred lines (RIL) from a population developed from a cross of N87-984-16 × TN93-99 were used. Ground soybean seed samples were analyzed for Met and Cys concentration using a near infrared spectroscopy instrument. Data were analyzed using SAS software and QTL Cartographer. RIL differed (P<0.01) in Met and Cys concentrations, with a range of 5.1–7.3 (g kg−1 seed dry weight) for Cys and 4.4–8.8 (g kg−1 seed dry weight) for Met. Heritability estimates on an entry mean basis were 0.14 and 0.57 for Cys and Met, respectively. A total of 94 polymorphic simple sequence repeat molecular genetic markers were screened in the RIL. Single factor ANOVA was used to identify candidate QTL, which were confirmed by composite interval mapping using QTL Cartographer. Four QTL linked to molecular markers Satt235, Satt252, Satt427 and Satt436 distributed on three molecular linkage groups (MLG) D1a, F and G were associated with Cys and three QTL linked to molecular markers Satt252, Satt564 and Satt590 distributed on MLG F, G and M were associated with Met concentration in soybean seed. QTL associated with Met and Cys in soybean seed will provide important information to breeders targeting improvements in the nutritional quality of soybean.



Similar content being viewed by others
References
Altenbach SB, Pearson KW, Leung FW, Sun SS (1987) Cloning and sequence analysis of a cdna encoding a brazil nut protein exceptionally rich in methionine. Plant Mol Biol 8:239–250
Altenbach SB, Pearson KW, Meeker G, Staraci LC, Sun SS (1989) Enhancement of the methionine content of seed proteins by the expression of a chimeric gene encoding a methionine-rich protein in transgenic plants. Plant Mol Biol 13:513–522
Brummer EC, Graef GL, Orf J, Wilcox JR, Shoemaker RC (1997) Mapping QTL for seed protein and oil content in eight soybean populations. Crop Sci 37:370–378
Burton JW, Carter TE, Wilson RF (1999) Registration of ‘prolina’ soybean. Crop Sci 39:294–295
Churchill GA, Doerge RW (1994) Empirical threshold values for quantitative trait mapping. Genetics 138:963–971
Clarke EJ, Wiseman J (2000) Developments in plant breeding for improved nutritional quality of soybeans I. Protein and amino acids content. J Agric Sci 134:111–124
Concibido VC, La Vallee B, McLaird P, Pineda N, Meyer J, Hummel L, Yang J, Wu K, Delannay X (2003) Introgression of a quantitative trait locus for yield from glycine soja into commercial soybean cultivars. Theor Appl Genet 106:575–582
Cregan PB, Jarvik T, Bush AL, Shoemaker RC, Lark KG, Kahler AL, Kaya N, VanToai TT, Lohnes DG, Chung J (1999) An integrated genetic linkage map of the soybean genome. Crop Sci 39:1464–1490
Csanadi G, Vollmann J, Stift G, Lelley T (2001) Seed quality qtls identified in a molecular map of early maturing soybean. Theor Appl Genet 103:912–919
Falconer DS, Mackay TFC (1996) Introduction to quantitative genetics, 4th edn. Longman Group Ltd, London
Fontaine J, Horr J, Schirmer B (2001) Near-infrared reflectance spectroscopy enables the fast and accurate prediction of the essential amino acid contents in soy, rapeseed meal, sunflower meal, peas, fish meal, meat meal products, and poultry meal. J Agric Food Chem 49:57–66
Friedman M, Brandon DL (2001) Nutritional and health benefits of soy proteins. J Agric Food Chem 49:1069–1086
George AA, de Lumen BO (1991) A novel methionine-rich protein in soybean seed: Identification, amino acid composition, and n-terminal sequence. J Agric Food Chem 39:224–227
Imsande J (2001) Selection of soybean mutants with increased concentrations of seed methionine and cysteine. Crop Sci 41:510–515
Imsande J, Schmidt JM (1998) Effect of n source during soybean pod filling on nitrogen and sulfur assimilation and remobilization. Plant Soil 202:41–47
Kearsey MJ, Pooni HS (1996) The genetical analysis of quantitative traits, 1st edn. Chapman and Hall, New York
Kortt AA, Caldwell JB (1990) Low molecular weight albumins from sunflower seed: identification of a methionine-rich. Phytochemistry 29:2805–2810
Kwanyuen P, Wilson RF, Burton JW (1998) Soybean protein quality. In: Koseoglu SS et al (eds) Oilseeds and edible oils processing, vol I. AOCS Press, Champaign, IL, pp 284–289
Kwanyuen P, Pantalone VR, Burton JW, Wilson RF (1997) A new approach to genetic alteration of soybean protein composition and quality. J Am Oil Chem Soc 74:983–987
Lincoln SE, Daly MJ, Lander ES (1993) Constructing genetic linkage maps with mapmaker version 3.: a tutorial and reference manual, 3rd edn. Whitehead Institute for Biomedical Research, Cambridge, MA
Liu SM, Masters DG (2003) Amino acid utilization for wool production. In: D’Mello JPF (ed) Amino acids in animal nutrition, 2nd edn. CABI Publishing, Cambridge, MA, pp 309–328
Loudelt O, Chaillou S, Merigout P, Talbotec J, Daniel-Vedele F (2003) Quantitative trait loci analysis of nitrogen use efficiency in Arabidopsis. Plant Physiol 131:345–358
Luck P, Lanier T, Daubert C, Wilson RF, Kwanyuen P (2001) Functionality and visoelastic behaviour of prolina soybean isolate. In: Wilson RF (ed) Oilseed processing and utilization. AOCS Press, Champaign, IL, pp 197–202
Matthews BF (1999) Lysine, threonine, and methionine biosynthesis. In: Singh BK (ed) Plant amino acids: biochemistry and biotechnology. Marcel Dekker Inc, New York, pp 205–225
Muntz K, Christov V, Saalbach G, Saalbach I, Waddel D, Pickardt T, Schieder O, Wustenhagen T (1998) Genetic engineering for high methionine grain legumes. Nahrung 42:125–127
Njiti VN, Meksem K, Iqbal MJ, Johnson JE, Kassem MA, Zobrist KF, Kilo VY, Lightfoot DA (2002) Common loci underlie field resistance to soybean sudden death syndrome in forrest, pyramid, essex, and douglas. Theor Appl Genet 104:294–300
Nordlee JA, Taylor SL, Twonsend JA, Thomas LA, Bush RK (1996) Identification of a brazil-nut allergen in transgenic soybeans. New Engl J Medicine 334:688–692
Nyquist WE (1991) Estimation of heritability and prediction of selection response in plant populations. Crit Rev Plant Sci 10:235–322
Olsen MS, Krone TL, Phillips RL (2003) Bsss53 as a donor source for increased whole-kernel methionine in maize: selection and evaluation of high-methionine inbreds and hybrids. Crop Sci 43:1634–1642
Orf JH, Diers BW, Boerma HR (2004) Genetic improvement: conventional and molecular-based strategies. In: Boerma HR, Specht JE (eds) Soybeans: improvement, production, and uses, 3rd edn. American Society of Agronomy, Madison, pp 417–450
Pantalone VR, Allen FL, Landau-Ellis D (2003) Registration of TN93–99 soybean germplasm. Crop Sci 43:1137
Panthee DR, Pantalone VR, Sams CE, Saxton AM, West DR, Rayford WE (2004a) Genomic regions governing soybean seed nitrogen accumulation. J Am Oil Chem Soc 81:77–81
Panthee DR, Kwanyuen P, Sams CE, West DR, Saxton AM, Pantalone VR (2004b) Quantitative trait loci for beta-conglycinin (7 s) and glycinin (11 s) fractions of soybean storage protein. J Am Oil Chem Soci 81:1005–1012
Ravanel S, Gakiere B, Job D, Douce R (1998) The specific features of methionine biosynthesis and metabolism in plants. Proc Nat Acad Sci USA 95:7805–7812
Rector BG, All JN, Parrott WA, Boerma HR (1998) Identification of molecular markers linked to quantitative trait loci for soybean resistance to corn earworm. Theor Appl Genet 96:786–790
Saito K (1999) Biosynthesis of cysteine. In: Singh BK (ed) Plant amino acids: Biochemistry and biotechnology. Marcel Dekker Inc, New York, pp 267–291
SAS Institute Inc (2002) Sas guide for personal computers, 9th edn. SAS Institute, Cary
Sexton PJ, Paek NC, Shibles RM (1998) Effects of nitrogen source and timing of sulfur deficiency on seed yield and expression of 11s and 7s seed storage proteins of soybean. Field Crops Res 59:1–8
Soytech Inc (2004) Oilseed statistics. Soytech Inc., Bar Harbor
Townsend JA, Thomas LA (1994) Factors which influence the Agrobacterium-mediated transformation of soybean. J Cell Biochem Suppl 18A:X1–014
Wang S, Basten CJ, Zeng ZB (2003) Windows qtl cartographer. Release V2.0. North Carolina State University, Raleigh, NC
Wang X, Stumpf DK, Larkins BA (2001a) Aspartate kinase 2. A candidate gene of a quantitative trait locus influencing free amino acid content in maize endosperm. Plant Physiol 125:1778–1787
Wang X, Won Y, Kim CS, Larkins BA (2001b) Quantitative trait locus mapping of loci influencing elongation factor 1a content in maize endosperm. Plant Physiol 125:1271–1282
Whitehead TF, Sapra VT, Rao DR (1989) Sulfur-containing amino acids and protein content in selected soybean progenies. Soybean Genet Newsl 16:74–80
Acknowledgements
Funding from the United Soybean Board in support of objectives of the Better Bean Initiative, and from the Tennessee Soybean Promotion Board is greatly appreciated. Continued support by the Tennessee Agriculture Experiment Station is also highly appreciated. We would like to express our thanks to Dr. Madeleine Spencer, Dr. David L. Hyten, Jr. and to Debbie Landau-Ellis for their contributions to this project.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by S. J. Knapp
Rights and permissions
About this article
Cite this article
Panthee, D.R., Pantalone, V.R., Sams, C.E. et al. Quantitative trait loci controlling sulfur containing amino acids, methionine and cysteine, in soybean seeds. Theor Appl Genet 112, 546–553 (2006). https://doi.org/10.1007/s00122-005-0161-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00122-005-0161-6