Abstract
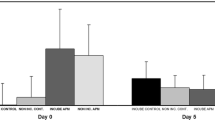
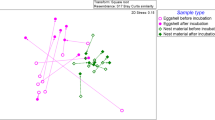
Avian incubation dramatically reduces the abundance and diversity of microbial assemblages on eggshells, and this effect has been hypothesized as an adaptive explanation for partial incubation, the bouts of incubation that some birds perform during the egg-laying period. However, the mechanisms for these antimicrobial effects are largely unknown. In this study, we hypothesized that microbial inhibition is partly achieved through removal of liquid water, which generally enhances microbial growth, from eggshells, and experimentally tested this hypothesis in two ways. First, we placed the first- and second-laid eggs of tree swallow (Tachycineta bicolor) clutches in unincubated holding nests with either ambient or increased water on eggshells. Second, we added water to eggshells in naturally partially incubated nests. We compared microbial growth on shells during a 5-day experimental period and found that, as predicted, both unincubated groups had higher microbial growth than naturally partially incubated controls, and that only in the absence of incubation did wetted eggs have higher microbial growth than unwetted eggs. Thus, we have shown that water increases microbial growth on eggshells and that incubation nullifies these effects, suggesting that removal of water from egg surfaces is one proximate mechanism for the antimicrobial effects of incubation.


Similar content being viewed by others
References
Amat JA, Masero JA (2007) The functions of belly-soaking in Kentish Plovers Charadrius alexandrinus. Ibis 149:91–97
Berger S, Disko R, Gwinner H (2003) Bacteria in starling nests. J Ornithol 144:317–322
Board RG, Fuller R (1974) Non-specific antimicrobial defences of avian egg embryo and neonate. Biol Rev 49:15–49
Board RG, Tranter HS (1995) The microbiology of eggs. In: Stadelman WJ, Cotterill OJ (eds) Egg science and technology. Haworth, Binghamton, pp 81–104
Bruce J, Drysdale EM (1991) Egg hygiene: routes of infection. In: Tullett SG (ed) Avian incubation. Butterworth Heinemann, Northampton, pp 257–276
Bruce J, Drysdale EM (1994) Trans-shell transmission. In: Board RG Fuller R (ed) Microbiology of the avian egg. Chapman and Hall, London, pp 63–91
Cook MI, Beissinger SR, Toranzos GA, Rodriguez RA, Arendt WJ (2003) Trans-shell infection by pathogenic micro-organisms reduces the shelf life of non-incubated bird’s eggs: a constraint on the onset of incubation? Proc R Soc Lond B Biol Sci 270:2233–2240
Cook MI, Beissinger SR, Toranzos GA, Arendt WJ (2005a) Incubation reduces microbial growth on eggshells and the opportunity for trans-shell infection. Ecol Lett 8:532–537
Cook MI, Beissinger SR, Toranzos GA, Rodriguez RA, Arendt WJ (2005b) Microbial infection affects egg viability and incubation behavior in a tropical passerine. Behav Ecol 16:30–36
Deeming DC (1995) Factors affecting hatchability during commercial incubation of ostrich (Struthio camelus) eggs. Br Poult Sci 36:51–65
Deeming DC (2002a) In: Deeming DC (ed) Avian incubation behaviour environment and evolution, vol 13, Oxford ornithology. Oxford University Press, Oxford
Deeming DC (2002b) Patterns and significance of egg turning. In: Deeming DC (ed) Avian incubation behaviour environment and evolution, vol 13, Oxford ornithology. Oxford University Press, Oxford, pp 161–178
Drent RH (1973) The natural history of incubation. In: Farner DS (ed) Breeding biology of birds. National Academy of Sciences, Washington, pp 262–332
Fierer N, Jackson RB (2006) The diversity and biogeography of soil bacterial communities. Proc Natl Acad Sci USA 103:626–632
Gill FB (1995) Nests and incubation. In: Gill FB (ed) Ornithology, 2nd edn. WH Freeman and Co., New York
Godard RD, Wilson M, Frick JW, Siegel PB, Bowers BB (2007) The effects of exposure and microbes on hatchability of eggs in open-cup and cavity nests. J Avian Biol 38:709–716
Heeb P, Kölliker M, Richner H (2000) Bird-ectoparasite interactions, nest humidity, and ectoparasite community structure. Ecology 81:958–968
Hilton GM, Hansell MH, Ruxton GD, Reid JM, Monaghan P (2004) Using artificial nests to test importance of nesting material and nest shelter for incubation energetics. Auk 121:777–787
Houston S, Saunders JR, Crawford RD (1997) Aerobic bacterial flora of addled raptor eggs in Saskatchewan. J Wildl Dis 33:328–331
Jagnow J, Clegg S (2003) Klebsiella pneumoniae MrkD-mediated biofilm formation on extracellular matrix- and collagen-coated surfaces. Microbiology 149:2397–2405
Jetz W, Sekercioglu CH, Bohning-Gaese K (2008) The worldwide variation in avian clutch size across species and space. PLoS Biol 6:e303
Kern MD, Cowie RJ (1995) Humidity levels in pied flycatcher nests measured using capsule hygrometers. Auk 112:564–570
Lack DL (1947) The significance of clutch size, parts 1 and 2. Ibis 89: 302–352
Madigan MT, Martinko JM, Dunlap PV, Clark DP (2005) Brock biology of microorganisms. Benjamin Cummings, New York
McComb WC, Noble RE (1981) Microclimates of nest boxes and natural cavities in Bottomland Hardwoods. J Wildl Manage 45:284–289
Messens W, Grijspeerdt K, Herman L (2005) Eggshell penetration by Salmonella: a review. World Poult Sci J 61:71–86
Patrick LB, Fraser LH, Kershener MW (2008) Large-scale manipulation of plant litter and fertilizer in a managed successional temperate grassland. Plant Ecol 197:183–195
Pinowski J, Barkowska M, Kruszewicz A, Kruszewicz A (1994) The causes of the mortality of eggs and nestlings of Passer sp. J Biosci 19:441–451
Rendell WB, Verbeek NAM (1996) Old nest material in nest boxes of tree swallows: effects on nest-site choice and nest building. Auk 113:319–328
Robertson RJ, Stutchbury BJ, Cohen RR (1992) Tree swallow (Tachycineta bicolor). In: Poole A (ed) The birds of North America online Ithaca. Cornell Lab of Ornithology, Cornell
Romanoff AL (1960) The avian embryo; structural and functional development. Macmillan, New York
Shawkey MD, Kosciuch KL, Liu M, Rohwer FC, Loos ER, Wang JM, Beissinger SR (2008) Do birds differentially distribute antimicrobial proteins within clutches of eggs? Behav Ecol 19:920–927
Shawkey MD, Firestone MK, Brodie EL, Beissinger SR (2009) Avian incubation inhibits growth and diversification of bacterial assemblages on eggs. PLoS ONE 4:e4522
Sparks N (1994) Shell accessory materials: structure and function. In: Board RG, Fuller R (eds) Microbiology of the avian egg. Chapman and Hall, London, pp 25–42
Stevens TO, Holbert BS (1995) Variability and density dependence of bacteria in terrestrial subsurface samples: implications for enumeration. J Microbiol Methods 21:283–292
Wang JM, Beissinger SR (2009) Variation in the onset of incubation and its influence on avian hatching success and asynchrony. Anim Behav 78:601–613
Wesołowski T, Czeszczewik D, Rowinski P, Walankiewicz W (2002) Nest soaking in natural holes—a serious cause of breeding failure? Ornis Fenn 79:138
White FN, Kinney JL (1974) Avian incubation. Science 186:107–115
Wilson HR (1991) Physiological requirements of the developing embryo: temperature and turning. In: Tullett SG (ed) Avian incubation. Butterworth-Heineman, London, pp 145–156
Zach R (1982) Hatching asynchrony egg size growth and fledging in tree swallows. Auk 99:695–700
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
D’Alba, L., Oborn, A. & Shawkey, M.D. Experimental evidence that keeping eggs dry is a mechanism for the antimicrobial effects of avian incubation. Naturwissenschaften 97, 1089–1095 (2010). https://doi.org/10.1007/s00114-010-0735-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00114-010-0735-2