Abstract
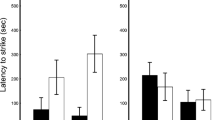
Caudal autotomy (tail shedding) is a defence mechanism against predation which is used by lizards when other tactics, such as crypsis and escape, prove ineffective. The speed at which autotomy occurs has important implications for survival, and the accuracy of tail loss is of consequence for an individual’s future fitness. Autotomy is shaped by both taxon-specific tail morphology as well as environmental factors such as predator history, and it can be difficult to distinguish between these processes. In this study, the frequency of tail-regeneration observed (field rate of autotomy), latency (speed of autotomy in the laboratory) and accuracy of tail loss were measured in six lizard species from two families (Scincidae and Diplodactylidae). The field rate and latency of autotomy was similar among all species except for the large nocturnal skink Oligosoma macgregori, which was less likely to autotomise. Latency and field rates of autotomy were not correlated, implying that the field rates of autotomy are related to predation attacks, social interactions, or some other environmental factor, rather than an innate disposition to autotomy. Further study, for example comparing populations with low and high predation pressure, will help to explain which of these factors are influencing autotomy rates.

Similar content being viewed by others
References
Armitage P (1971) Statistical methods in medical research. Blackwell, Oxford
Arnold EN (1984) Evolutionary aspects of tail shedding in lizards and their relatives. J Nat Hist 18:127–169
Arnold EN (1988) Caudal autotomy as a defense. In: Gans C, Huey RB (eds) Biology of the Reptilia: vol. 16, Defense and Life History. Alan R. Liss, Inc, New York, p 659
Ballinger RE, Tinkle DW (1979) On the cost of tail regeneration to body growth in lizards. J Herpetol 13:374–375
Bateman PW, Fleming PA (2009) To cut a long tail short: a review of lizard caudal autotomy studies carried out over the last 20 years. J Zool (Lond.) 277:1–14
Bauer AM, Russell AP (1994) Is autotomy frequency reduced in geckos with 'actively functional' tails? Herpetol Nat Hist 2:1–15
Bellairs ADA, Bryant SV (1985) Autotomy and regeneration in reptiles. In: Gans C, Billet F (eds) Biology of the Reptilia. Wiley, New York, pp 301–410
Chapple DG, Swain R (2002) Distribution of energy reserves in a viviparous skink: does tail autotomy involve the loss of lipid stores? Austral Ecol 27:565–572
Chapple DG, McCoull CJ, Swain R (2002) Changes in reproductive investment following caudal autotomy in viviparous skinks (Niveoscincus metallicus): lipid depletion or energetic diversion? J Hertpetol 36:480–486
Chapple DG, McCoull CJ, Swain R (2004) Effect of tail loss on sprint speed and growth in newborn skinks, Niveoscincus metallicus. J Herpetol 38:137–140
Chapple DG, Ritchie PA, Daugherty CH (2009) Origin, diversification, and systematics of the New Zealand skink fauna (Reptilia: Scincidae). Mol Phylogen Evol 522:470–487
Cooper WE Jr, Pérez-Mellado V, Vitt LJ (2004) Ease and effectiveness of costly autotomy vary with predation intensity among lizard populations. J Zool (Lond.) 262:243–255
Daniels CB (1984) The importance of caudal lipid in the gecko Phyllodactylus marmoratus. Herpetologica 40:337–344
Dial BE (1978) Aspects of the behavioural ecology of two Chihahaun Desert geckos (Reptilia, Lacertilia, Gekkonidae). J Herpetol 12:209–216
Dial BE, Fitzpatrick LC (1984) Predator escape success in tailed versus tailless Scincella lateralis (Sauria: Scincidae). Anim Behav 32:301–302
Fox SF, Rostker MA (1982) Social cost of tail loss in Uta stansburiana. Science 218:692–693
Hardy GS (1977) The New Zealand Scincidae (Reptilia: Lacertilia): a taxonomic and zoogeographic study. N Z J Zool 4:221–325
Hare KM (2007) Hoplodactylus chrysosireticus (Goldstripe gecko). Body temperature. Herp Rev 38:456–457
Hare KM, Miller JH, Clark A, Daugherty CH (2005) Total lactate dehydrogenase activity of tail muscle is not cold-adapted in nocturnal lizards from cool-temperate habitats. Comp Biochem Physiol Part B 142:438–444
Hoare JM, Adams LK, Bull LS, Towns DR (2007a) Attempting to manage complex predator—prey interactions fails to avert imminent extinction of a threatened New Zealand skink population. J Wildl Manag 71:1576–1584
Hoare JM, Pledger S, Nelson NJ, Daugherty CH (2007b) Avoiding aliens: behavioural plasticity in habitat use enables large, nocturnal geckos to survive Pacific rat invasions. Biol Conserv 136:510–519
Johnson CR (1970) Escape behavior and camouflage in two subspecies of Sceloporus occidentalis. Am Mid Nat 84:280–282
Langkilde T, Shine R (2006) How much stress do researchers inflict on their study animals? A case study using a scincid lizard, Eulamprus heatwolei. J Exp Biol 209:1035–1043
Martín J, Salvador A (1993) Tail loss reduces mating success in the Iberian rock-lizard, Lacerta monticola. Behav Ecol Sociobiol 32:185–189
McConnachie S, Whiting MJ (2003) Costs associated with tail autotomy in an ambush foraging lizard, Cordylus melanotus melanotus. Afr Zool 38:57–65
Miskelly C (1999) Mana Island ecological restoration plan. Wellington Conservancy, Department of Conservation, Wellington
Newman DG (1994) Effects of a mouse, Mus musculus, eradication and habitat change on lizard populations of Mana Island, New Zealand, with special reference to McGregor's skink, Cyclodina macgregori. N Z J Zool 21:443–456
Pafilis P, Foufopoulos J, Poulakakis N, Lymberakis P, Valakos ED (2009) Tail shedding in island lizards (Lacertidae, Reptilia): decline of antipredator defences in relaxed predation environments. Evolution 63:1262–1278
R-Development-Core-Team (2008) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna
Towns DR, Daugherty CH (1994) Patterns of range contractions and extinctions in the New Zealand herpetofauna following human colonisation. N Z J Zool 21:325–339
Turner FB, Medica PA, Jennrich RI, Maza BG (1982) Frequencies of broken tails among Uta stansburiana in southern Nevada and a test of the predation hypothesis. Copeia 1982:835–840
Vitt LJ (1983) Tail loss in lizards: the significance of foraging and predator escape modes. Herpetologica 39:151–162
Vitt LJ, Zani PA (1997) Ecology of the nocturnal lizard Thecadactylus rapicauda (Sauria: Gekkonidae) in the Amazon Region. Herpetologica 53:165–179
Wallis GP, Trewick SA (2009) New Zealand phylogeography: evolution on a small continent. Mol Ecol 18:3548–3580
Werner YL, Whitaker AH (1978) Observations and comments on the body temperatures of some New Zealand reptiles. N Z J Zool 5:375–393
Acknowledgements
Thanks to those who assisted with lizard capture in the field, C Miskelly for McGregor’s skink capture information, and JM Hoare, AR Martin, and three anonymous reviewers for critical comments on the draft. Financial support was provided by a VUW Postgraduate Scholarship and a Foundation for Research Science and Technology New Zealand Science and Technology Postdoctoral Fellowship. Research was carried out with approval of the New Zealand Department of Conservation (LIZ0406, 9/375ROA), VUW Animal Ethics Committee (2002R3, 2004R2), and consultation with Ngāti Toa people.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hare, K.M., Miller, K.A. Frequency of tail loss does not reflect innate predisposition in temperate New Zealand lizards. Naturwissenschaften 97, 197–203 (2010). https://doi.org/10.1007/s00114-009-0628-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00114-009-0628-4