Abstract
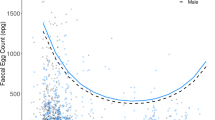
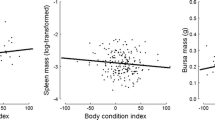
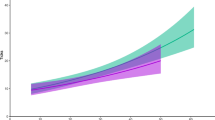
We analyzed the relationships among spleen size, body condition (measured as kidney fat), and larval counts of the nematode Elaphostrongylus cervi in red deer (Cervus elaphus). The aim was to investigate the interaction between host body condition and intensity of infection with parasites. As red deer are highly polygynous, we also tested whether these relationships varied with sex and age of the hosts. Kidney fat and spleen size were positively correlated in subadults (2–3 years old) and adults (>3 years old), but not in calves (<1 year old) or yearlings (1–2 years old). Spleen size was negatively associated with nematode load in subadult females and in adult males. These two age classes are potentially the most nutritionally stressed, as subadult hinds are still growing and often engaging in rearing their first calf, and adult stags were sampled just after the rut, which is recognized as a substantial energy drain in this age–sex class, as they compete to hold females during the mating season. Body condition related negatively to parasite count only in adult males. In the context of red deer life history, these findings suggest that spleen size is dependent on body condition and that it could be affected by variation in resource partitioning among immune defense, growth, and reproductive effort in red deer. For the first time in a wild mammal, the spleen mass is shown to be positively related to body condition and negatively related to parasite infection. We conclude that elucidating whether spleen mass reflects immune defense investment or a measure of general body condition should contribute to understanding topical issues in mammal ecology.


Similar content being viewed by others
References
Albon SD, Mitchell B, Staines BW (1983) Fertility and body weight in female red deer: a density-dependent relationship. J Anim Ecol 52:969–980
Alexander J, Stimson WH (1989) Sex hormones and the course of parasitic infection. Parasitol Today 4:189–193
Bradley JE, Jackson JA (2004) Immunity, immunoregulation and the ecology of trichuriasis and ascariasis. Parasite Immunol 26:429–441
Burnham KP, Anderson DR (1998) Model selection and inference: a practical information-theoretical approach. Springer, Berlin
Carranza J, Alarcos S, Sánchez-Prieto CB, Valencia J, Mateos C (2004) Disposable-soma senescence mediated by sexual selection in an ungulate. Nature 432:215–218
Coop RL, Kyriazakis I (1999) Nutrition–parasite interaction. Vet Parasitol 84:187–204
Coop RL, Kyriazakis I (2001) Influence of host nutrition on the development and consequences of nematode parasitism in ruminants. Trends Parasitol 17:325–330
Clutton-Brock TH, Guinness FE, Albon SP (1982) Reed deer: behaviour and ecology of two sexes. University of Chicago Press, Chicago
Clutton-Brock TH, Albon SP, Guinnes FE (1984) Fitness cost of gestation and lactation in wild mammals. Nature 337:260–262
Clutton-Brock TH, Albon SD, Guinness FE (1988) Reproductive success in male and female red deer. In: Clutton-Brock TH (ed) Reproductive success. University of Chicago Press, Chicago, pp 325–343
Crawley MJ (1993) GLIM for ecologists. Blackwell, Oxford
Festa-Bianchet M (1998) Condition-dependent reproductive success in bighorn ewes. Ecol Lett 1:91–94
Finger SE, Brisbin IL Jr, Smith MH (1981) Kidney fat as a predictor of body condition in white-tailed deer. J Wildl Manage 45:964–968
Folstad I, Karter AJ (1992) Parasites, bright males, and the immunocompetence handicap. Am Nat 139:603–622
Forrester SG, Lankester MW (1997) Extracting protostrongylid nematode from ungulate faeces. J Wildl Dis 33:511–516
Gaillard JM, Festa-Bianchet M, Delorme D, Jorgenson J (2000) Body mass and individual fitness in female ungulates, bigger is not always better. Proc R Soc Lond B 267:471–477
Garside P, Behnke JM, Rose RA (1989) The immune response of male DSN hamsters to a primary infection with Ancylostoma ceylanicum. J Helminthol 63:251–260
Gaudernack G, Halvorsen O, Skorping A, Stokkan KA (1984) Humoral immunity and output of first-stage lamrvae of Elaphostrongylus rangiferi (Nematoda, Metastrongyloidea) by infected reindeer, Rangifer tarandus tarandus. J Helminthol 58:13–18
Handeland K, Gibbons LM, Skorping A (2000) Aspects of the life cycle and pathogenesis of Elaphostrongylus cervi in red deer (Cervus elaphus). J Parasitol 86:1061–1066
John JL (1994) The avian spleen: a neglected organ. Q Rev Biol 69:327–351
John JL (1995) Parasites and the avian spleen. Biol J Linn Soc 54:87–106
Johns PE, Smith MH, Chesser RK (1984) Annual cycles of the kidney fat index in a southeastern white-tailed deer herd. J Wildl Manage 48:969–973
Hõrak P, Tummeleht L, Talvik H (2006) Predictors and markers of resistance to neurotropic nematode infection in rodent host. Parasitol Res 98:396–402
Klevezal GA, Kleinenberg SE (1967) Age determination of mammals from annual layers in teeth and bones. Clearinghouse Fed Sci Tech Inf US Dep Commer, Springfield
Kopp WC (1990) The immune functions of the spleen. In: Bowdler AJ (ed) The spleen: structure, function and clinical significance. Chapman & Hall Medical, London, pp 103–126
Landete-Castillejos T, Gortazar C, Vicente J, Fierro Y, García A, Gallego L (2004) Age-related foetal sex ratio bias in Iberian red deer (Cervus elaphus hispanicus): are male calves too expensive for growing mothers? Behav Ecol Sociobiol 56:1–8
Lochmiller RL, Deeremberg C (2000) Trade-offs in evolutionary immunology: just what is the cost of immunity? Oikos 88:87–98
Møller AP, Saino N (2004) Immune response and survival. Oikos 104:299–304
Møller AP, Christe PH, Erritzoe J, Mavarez A (1998a) Condition, disease and immune defence. Oikos 83:301–306
Møller AP, Dufva R, Erritzoe J (1998b) Host immune function and sexual selection in birds. J Evol Biol 11:703–719
Moore SL, Wilson K (2002) Parasites as a viability cost of sexual selection in natural populations of mammals. Science 297:2015–2017
Morand S, Poulin R (2000) Nematode parasite species richness and the evolution of spleen size in birds. Can J Zool 78:1356–1360
Mitchell RJ (1992) Testing evolutionary and ecological hypothesis path analysis and structural equation modelling. Funct Ecol 6:123–129
Mulvey M, Aho JM (1993) Parasitism and mate competition: liver flukes in white-tailed deer. Oikos 66:187–192
Nunn CL (2002) Spleen size, disease risk and sexual selection: a comparative study in primates. Evol Ecol Res 4:91–107
Owensmith N (1984) Spatial and temporal components of the mating systems of kudu bulls and red deer stags. Anim Behav 32:321–332
Pelletier F, Page KA, Ostiguy T, Festa-Bianchet M (2005) Faecal counts of lungworm larvae and reproductive effort in bighorn sheep, Ovis canadensis. Oikos 110:473–480
Promislow D, Harvey P (1990) Living fast and dying young: a comparative analysis of life-history variation among mammals. J Zool 220:417–437
Rantala MJ, Roff DA (2005) An analysis of trade-off in immune function, body size and development time in the Mediterranean field cricket, Gryllus bimaculatus. Funct Ecol 19:323–330
Reilly FD (1985) Innervation and vascular pharmacodynamics of the mammalian spleen. Cell Mol Life Sci 41:187–192
Schalk G, Forbes MR (1997) Male biases in parasitism of mammals: effects of study type, host age, and parasite taxon. Oikos 78:67–74
Smith KG, Hunt JL (2004) On the use of spleen mass as a measure of avian immune system strength. Oecologia 138:28–31
Shutler D, Alisauskas RT, McLaughlin JD (1999) Mass dynamics of the spleen and other organs in geese: measures of immune relationships to helminths? Can J Zool 77:351–359
Strain SA, Stear MJ (2001) The influence of protein supplementation on the immune response to Haemonchus contortus. Parasite Immunol 23:527–531
Vicente J, Gortazar C (2001) High prevalence of large spiny-tailed protostrongylid larvae in Iberian red deer. Vet Parasitol 96:165–170
Vicente J, Fierro Y, Gortazar C (2005) Seasonal dynamics of the fecal excretion of Elaphostrongylus cervi (Nematoda, Metastrongyloidea) first-stage larvae in Iberian red deer (Cervus elaphus hispanicus) from south Spain. Parasitol Res 95:60–64
Vicente J, Fernández de Mera IG, Gortazar C (2006) Epidemiology and risk factors analysis of elaphostrongylosis in red deer (Cervus elaphus) from Spain. Parasitol Res 98:77–85
Vincent AL, Ash LR (1978) Splenomegaly in jirds (Meriones unguiculatus) infected with Brugia malayi (Nematoda: Filaroidea) and related species. Am J Trop Med Hyg 27:514–520
Watkins RA, Moshier SE, Odell WD, Pinter AJ (1991) Splenomegaly and reticulocytosis caused by Babesia microti infections in natural populations of the montane vole, Microtus montanus. J Protozool 38:573–576
Watson TG (1983) Some clinical and parasitological features of Elaphostrongylus cervi infections in Cervus elaphus. N Z J Zool 10:129
Watson TG (1984) Tissue worm in red deer. Symptoms and control. In: AgLink farm production and practice 249. Ministry of Agriculture and Food, Wellington, New Zealand
Wilson K, Grenfell BT (1997) Generalised linear modelling for parasitologists. Parasitol Today 13:33–38
Wirtz P, Oldekop G (1991) Time budgets of waterbuck (Kobus ellipsiprymnus) of different age, sex and social status. International Journal of Mammalian Biology 56:48–58
Zuk M (1990) Reproductive strategies and sex differences in disease susceptibility: an evolutionary viewpoint. Parasitol Today 6:231–233
Zuk M, Stoehr AM (2002) Immune defence and host life history. Am Nat 160: S9–S2
Acknowledgments
The authors thank J. Martínez-Padilla, S. Redpath, J. Viñuela, and N. Walker for their comments on earlier versions of the manuscript and/or statistical advice. We are very grateful to J. Irvine whose comments and grammar corrections improved the manuscript. We also are very grateful to anonymous reviewers who greatly improved the manuscript. This study was supported by project AGL2005-07401, Ministerio de Educación. L. Pérez-Rodríguez and J. Vicente had a grant from Ministerio de Educación y Ciencia and Junta de Comunidades de Castilla-La Mancha, respectively. This is also a contribution to the agreement between FG-UCLM and Grupo Santander and to the agreement between Yolanda Fierro and Universidad de Castilla-La Mancha. The authors declare that this study complies with current Spanish law.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Vicente, J., Pérez-Rodríguez, L. & Gortazar, C. Sex, age, spleen size, and kidney fat of red deer relative to infection intensities of the lungworm Elaphostrongylus cervi . Naturwissenschaften 94, 581–587 (2007). https://doi.org/10.1007/s00114-007-0231-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00114-007-0231-5