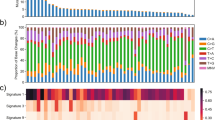
Abstract
Our previous extensive analysis revealed a significant proportion of early-onset colorectal tumors from India to be localized to the rectum in younger individuals and devoid of deregulated Wnt/β-catenin signaling. In the current study, we performed a comprehensive genome-wide analysis of clinically well-annotated microsatellite stable early-onset sporadic rectal cancer (EOSRC) samples. Results revealed extensive DNA copy number alterations in rectal tumors in the absence of deregulated Wnt/β-catenin signaling. More importantly, transcriptome profiling revealed a (non-Wnt/β-catenin, non-MSI) genetic signature that could efficiently and specifically identify Wnt− rectal cancer. The genetic signature included a significant representation of genes belonging to Ca2+/NFAT signaling pathways that were validated in additional samples. The validated NFAT target genes exhibited significantly higher expression levels than canonical Wnt/β-catenin targets in Wnt− samples, an observation confirmed in other CRC expression data sets as well. We confirmed the validated genes to be transcriptionally regulated by NFATc1 by (a) evaluating their respective transcript levels and (b) performing promoter-luciferase and chromatin immunoprecipitation assays following ectopic expression as well as knockdown of NFATc1 in CRC cells. NFATc1 and its targets RUNX2 and GSN could drive increased migration in CRC cells. Finally, the validated genes were associated with poor survival in the cancer genome atlas CRC expression data set. This study is the first comprehensive molecular characterization of EOSRC that appears to be driven by noncanonical tumorigenesis pathways.
Key messages
-
Early-onset sporadic rectal cancer exhibits DNA gain and loss without Wnt activation.
-
Ca2+/NFAT signaling appears to be activated in the absence of Wnt activation.
-
An eight-gene genetic signature distinguishes Wnt+ and Wnt− rectal tumors.
-
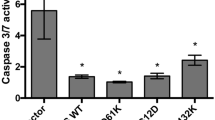
NFAT and its target genes regulate tumorigenic properties in CRC cells.





Similar content being viewed by others
References
Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D (2011) Global cancer statistics. CA Cancer J Clin 61:69–90
Polakis P (2007) The many ways of Wnt in cancer. Curr Opin Genet Dev 17:45–51
de la Chapelle A, Hampel H (2010) Clinical relevance of microsatellite instability in colorectal cancer. J Clin Oncol 28:3380–3387
Shen L, Toyota M, Kondo Y, Lin E, Zhang L, Guo Y, Hernandez NS, Chen X, Ahmed S, Konishi K et al (2007) Integrated genetic and epigenetic analysis identifies three different subclasses of colon cancer. Proc Natl Acad Sci U S A 104:18654–18659
Ramnath T, Nandakumar A (2011) Estimating the burden of cancer. Natl Med J India 24:69–71
Gupta S, Bhattacharya D, Acharya AN, Majumdar S, Ranjan P, Das S (2010) Colorectal carcinoma in young adults: a retrospective study on Indian patients: 2000–2008. Color Dis 12:e182–e189
Heys SD, O'Hanrahan TJ, Brittenden J, Eremin O (1994) Colorectal cancer in young patients: a review of the literature. Eur J Surg Oncol 20:225–231
Meza R, Jeon J, Renehan AG, Luebeck EG (2010) Colorectal cancer incidence trends in the United States and United kingdom: evidence of right- to left-sided biological gradients with implications for screening. Cancer Res 70:5419–5429
Birkenkamp-Demtroder K, Olesen SH, Sorensen FB, Laurberg S, Laiho P, Aaltonen LA, Orntoft TF (2005) Differential gene expression in colon cancer of the caecum versus the sigmoid and rectosigmoid. Gut 54:374–384
TCGA (2012) Comprehensive molecular characterization of human colon and rectal cancer. Nature 487: 330–337
Frattini M, Balestra D, Suardi S, Oggionni M, Alberici P, Radice P, Costa A, Daidone MG, Leo E, Pilotti S et al (2004) Different genetic features associated with colon and rectal carcinogenesis. Clin Cancer Res 10:4015–4021
Lee YC, Lee YL, Chuang JP, Lee JC (2013) Differences in survival between colon and rectal cancer from SEER data. PLoS One 8:e78709
Slattery ML, Curtin K, Wolff RK, Boucher KM, Sweeney C, Edwards S, Caan BJ, Samowitz W (2009) A comparison of colon and rectal somatic DNA alterations. Dis Colon Rectum 52:1304–1311
Raman MDBR (2013) Molecular origins of colon and rectal cancer: not a Wnt-Wnt situation. Curr Colorectal Cancer Rep 9:365–371
Raman R, Kotapalli V, Adduri R, Gowrishankar S, Bashyam L, Chaudhary A, Vamsy M, Patnaik S, Srinivasulu M, Sastry R et al (2014) Evidence for possible non-canonical pathway(s) driven early-onset colorectal cancer in India. Mol Carcinog 53(Suppl 1):E181–E186
Kim YH, Pollack JR (2009) Comparative genomic hybridization on spotted oligonucleotide microarrays. Methods Mol Biol 556:21–32
Kwei KA, Bashyam MD, Kao J, Ratheesh R, Reddy EC, Kim YH, Montgomery K, Giacomini CP, Choi YL, Chatterjee S et al (2008) Genomic profiling identifies GATA6 as a candidate oncogene amplified in pancreatobiliary cancer. PLoS Genet 4:e1000081
Raman R, Kongara R, Kotapalli V, Gowrishankar S, Sastry RA, Nagari B, Bashyam MD (2014) Pathological stage significantly predicts survival in colorectal cancer patients: a study from two tertiary care centers in India. Colorectal Cancer 3:265–275
Verhaak RG, Tamayo P, Yang JY, Hubbard D, Zhang H, Creighton CJ, Fereday S, Lawrence M, Carter SL, Mermel CH et al (2013) Prognostically relevant gene signatures of high-grade serous ovarian carcinoma. J Clin Invest 123:517–525
Dejmek J, Safholm A, Kamp Nielsen C, Andersson T, Leandersson K (2006) Wnt-5a/Ca2+-induced NFAT activity is counteracted by Wnt-5a/Yes-Cdc42-casein kinase 1alpha signaling in human mammary epithelial cells. Mol Cell Biol 26:6024–6036
Korinek V, Barker N, Morin PJ, van Wichen D, de Weger R, Kinzler KW, Vogelstein B, Clevers H (1997) Constitutive transcriptional activation by a beta-catenin-Tcf complex in APC−/− colon carcinoma. Science 275:1784–1787
Yiu GK, Kaunisto A, Chin YR, Toker A (2011) NFAT promotes carcinoma invasive migration through glypican-6. Biochem J 440:157–166
Semenov MV, Habas R, Macdonald BT, He X (2007) SnapShot: noncanonical Wnt signaling pathways. Cell 131:1378
Basu M, Roy SS (2013) Wnt/beta-catenin pathway is regulated by PITX2 homeodomain protein and thus contributes to the proliferation of human ovarian adenocarcinoma cell, SKOV-3. J Biol Chem 288:4355–4367
Segditsas S, Tomlinson I (2006) Colorectal cancer and genetic alterations in the Wnt pathway. Oncogene 25:7531–7537
Marisa L, de Reynies A, Duval A, Selves J, Gaub MP, Vescovo L, Etienne-Grimaldi MC, Schiappa R, Guenot D, Ayadi M et al (2013) Gene expression classification of colon cancer into molecular subtypes: characterization, validation, and prognostic value. PLoS Med 10:e1001453
De Sousa EMF, Wang X, Jansen M, Fessler E, Trinh A, de Rooij LP, de Jong JH, de Boer OJ, van Leersum R, Bijlsma MF et al (2013) Poor-prognosis colon cancer is defined by a molecularly distinct subtype and develops from serrated precursor lesions. Nat Med 19:614–618
Sadanandam A, Lyssiotis CA, Homicsko K, Collisson EA, Gibb WJ, Wullschleger S, Ostos LC, Lannon WA, Grotzinger C, Del Rio M et al (2013) A colorectal cancer classification system that associates cellular phenotype and responses to therapy. Nat Med 19:619–625
Buchholz M, Schatz A, Wagner M, Michl P, Linhart T, Adler G, Gress TM, Ellenrieder V (2006) Overexpression of c-myc in pancreatic cancer caused by ectopic activation of NFATc1 and the Ca2+/calcineurin signaling pathway. EMBO J 25:3714–3724
Hassin-Baer S, Laitman Y, Azizi E, Molchadski I, Galore-Haskel G, Barak F, Cohen OS, Friedman E (2009) The leucine rich repeat kinase 2 (LRRK2) G2019S substitution mutation. Association with Parkinson disease, malignant melanoma and prevalence in ethnic groups in Israel. J Neurol 256:483–487
Li XQ, Du X, Li DM, Kong PZ, Sun Y, Liu PF, Wang QS, Feng YM (2015) ITGBL1 is a Runx2 transcriptional target and promotes breast cancer bone metastasis by activating the TGFbeta signaling pathway. Cancer Res 75:3302–3313
Deng B, Fang J, Zhang X, Qu L, Cao Z, Wang B (2015) Role of gelsolin in cell proliferation and invasion of human hepatocellular carcinoma cells. Gene 571:292–297
Fromigue O, Hay E, Barbara A, Marie PJ (2010) Essential role of nuclear factor of activated T cells (NFAT)-mediated Wnt signaling in osteoblast differentiation induced by strontium ranelate. J Biol Chem 285:25251–25258
Tripathi P, Wang Y, Coussens M, Manda KR, Casey AM, Lin C, Poyo E, Pfeifer JD, Basappa N, Bates CM et al (2014) Activation of NFAT signaling establishes a tumorigenic microenvironment through cell autonomous and non-cell autonomous mechanisms. Oncogene 33:1840–1849
Neal JW, Clipstone NA (2003) A constitutively active NFATc1 mutant induces a transformed phenotype in 3T3-L1 fibroblasts. J Biol Chem 278:17246–17254
Crabtree GR, Olson EN (2002) NFAT signaling: choreographing the social lives of cells. Cell 109(Suppl):S67–S79
Grumolato L, Liu G, Mong P, Mudbhary R, Biswas R, Arroyave R, Vijayakumar S, Economides AN, Aaronson SA (2010) Canonical and noncanonical Wnts use a common mechanism to activate completely unrelated coreceptors. Genes Dev 24:2517–2530
Vig M, Kinet JP (2009) Calcium signaling in immune cells. Nat Immunol 10:21–27
Sears CL, Garrett WS (2014) Microbes, microbiota, and colon cancer. Cell Host Microbe 15:317–328
Grivennikov SI (2013) Inflammation and colorectal cancer: colitis-associated neoplasia. Semin Immunopathol 35:229–244
Peuker K, Muff S, Wang J, Kunzel S, Bosse E, Zeissig Y, Luzzi G, Basic M, Strigli A, Ulbricht A et al (2016) Epithelial calcineurin controls microbiota-dependent intestinal tumor development. Nat Med 22:506–515
Gerlach K, Daniel C, Lehr HA, Nikolaev A, Gerlach T, Atreya R, Rose-John S, Neurath MF, Weigmann B (2012) Transcription factor NFATc2 controls the emergence of colon cancer associated with IL-6-dependent colitis. Cancer Res 72:4340–4350
Laskar RS, Talukdar FR, Mondal R, Kannan R, Ghosh SK (2014) High frequency of young age rectal cancer in a tertiary care centre of southern Assam, North East India. Indian J Med Res 139:314–318
Goh KL, Quek KF, Yeo GT, Hilmi IN, Lee CK, Hasnida N, Aznan M, Kwan KL, Ong KT (2005) Colorectal cancer in Asians: a demographic and anatomic survey in Malaysian patients undergoing colonoscopy. Aliment Pharmacol Ther 22:859–864
Bashyam MD, Kotapalli V, Raman R, Chaudhary AK, Yadav BK, Gowrishankar S, Uppin SG, Kongara R, Sastry RA, Vamsy M et al (2015) Evidence for presence of mismatch repair gene expression positive Lynch syndrome cases in India. Mol Carcinog 54:1807–1814
Acknowledgements
We are grateful to the patients and normal subjects for kindly agreeing to be a part of this study. We thank Dr. AS Raju, Research Associate, Laboratory of Molecular Oncology, CDFD for helping in microarray data analysis and for critical reading of the manuscript. The authors gratefully acknowledge the support from surgeons Dr. Mohana Vamsy, Dr. T Subramanyeshwar Rao, Dr. Sujit Patnaik, and Dr. KVVN Raju, Basavatarakam Indo-American Cancer Hospital and Research Institute, Hyderabad, India; Prof Mukta Srinivasulu, MNJ Institute of Oncology and Regional Cancer Centre, Hyderabad, India; and Dr. N Bheerappa, Nizam’s Institute of Medical Sciences, Hyderabad, India; and from pathologists Dr. I Satish Rao, Krishna Institute of Medical Sciences, Hyderabad, India; Dr. Sudha Murthy, Basavatarakam Indo-American Cancer Hospital and Research Institute, Hyderabad, India; and Dr. K T Vijaya, Care Hospitals, Hyderabad, India, for evaluating FFPE and IHC sections. We thank Ms. Leena Bashyam and Prof Aparna Dutta Gupta, Genomics Facility, School of Life Sciences, University of Hyderabad, Hyderabad, India, for providing access to the Agilent scanner.
Funding
This work was supported by an NIH-FIRCA grant (TW007963) to JP and MDB and two grants from the Department of Biotechnology, Government of India (BT/PR8609/MED/12/626/2013 and the National Bioscience Award project) to MDB. RK and RR, registered PhD students of Manipal University, are grateful to the Council of Scientific and Industrial Research, Government of India, for junior and senior research fellowships.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The study was performed as per the revised Helsinki declaration following approval of ethics committee of hospitals from where samples were collected as well as of the CDFD bioethics committee. Informed consent was obtained from patients.
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
ESM 1
(PDF 1724 kb)
Rights and permissions
About this article
Cite this article
Kumar, R., Raman, R., Kotapalli, V. et al. Ca2+/nuclear factor of activated T cells signaling is enriched in early-onset rectal tumors devoid of canonical Wnt activation. J Mol Med 96, 135–146 (2018). https://doi.org/10.1007/s00109-017-1607-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00109-017-1607-4