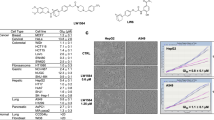
Abstract
Mitochondrial respiration is required for hypoxia-inducible factor (HIF)-1α stabilization, which is important for tumor cell survival, proliferation, and angiogenesis. Herein, small molecules that inhibit HIF-1α protein stability by targeting mitochondrial energy production were screened using the Library of Pharmacologically Active Compounds and cell growth assay in galactose or glucose medium. NNC 55-0396, a T-type Ca2+ channel inhibitor, was selected as a hit from among 1,280 small molecules. NNC 55-0396 suppressed mitochondrial reactive oxygen species-mediated HIF-1α expression as well as stabilization by inhibiting protein synthesis in a dose-dependent manner. NNC 55-0396 inhibited tumor-induced angiogenesis in vitro and in vivo by suppressing HIF-1α stability. Moreover, NNC 55-0396 significantly suppressed glioblastoma tumor growth in a xenograft model. Thus, NNC 55-0396, a small molecule targeting T-type Ca2+ channel, was identified by the systemic cell-based assay and was shown to have antiangiogenic activity via the suppression of HIF-1α signal transduction. These results provide new insights into the biological network between ion channel and HIF-1α signal transduction.
Key message
-
HIF-1α overexpression has been demonstrated in hypoxic cancer cells.
-
NNC 55-0396, a T-type Ca2+ channel inhibitor, inhibited HIF-1α expression via both proteasomal degradation and protein synthesis pathways.
-
T-type Ca2+ channel inhibitors block angiogenesis by suppressing HIF-1α stability and synthesis.
-
NNC 55-0396 could be a potential therapeutic drug candidate for cancer treatment.






Similar content being viewed by others
References
Wang GL, Semenza GL (1995) Purification and characterization of hypoxia-inducible factor 1. J Biol Chem 270:1230–1237
Mazure NM, Brahimi-Horn MC, Berta MA, Benizri E, Bilton RL, Dayan F, Ginouves A, Berra E, Pouyssegur J (2004) HIF-1: master and commander of the hypoxic world. A pharmacological approach to its regulation by siRNAs. Biochem Pharmacol 68:971–980
Masson N, Ratcliffe PJ (2003) HIF prolyl and asparaginyl hydroxylases in the biological response to intracellular O(2) levels. J Cell Sci 116:3041–3049
Zelzer E, Levy Y, Kahana C, Shilo BZ, Rubinstein M, Cohen B (1998) Insulin induces transcription of target genes through the hypoxia-inducible factor HIF-1alpha/ARNT. EMBO J 17:5085–5094
Thornton RD, Lane P, Borghaei RC, Pease EA, Caro J, Mochan E (2000) Interleukin 1 induces hypoxia-inducible factor 1 in human gingival and synovial fibroblasts. Biochem J 350(Pt 1):307–312
Kuo HP, Lee DF, Xia W, Wei Y, Hung MC (2009) TNFalpha induces HIF-1alpha expression through activation of IKKbeta. Biochem Biophys Res Commun 389:640–644
Li J, Davidson G, Huang Y, Jiang BH, Shi X, Costa M, Huang C (2004) Nickel compounds act through phosphatidylinositol-3-kinase/Akt-dependent, p70(S6k)-independent pathway to induce hypoxia inducible factor transactivation and Cap43 expression in mouse epidermal Cl41 cells. Cancer Res 64:94–101
Kwon HJ (2006) Discovery of new small molecules and targets towards angiogenesis via chemical genomics approach. Curr Drug Targets 7:397–405
Harding MW, Galat A, Uehling DE, Schreiber SL (1989) A receptor for the immunosuppressant FK506 is a cis-trans peptidyl-prolyl isomerase. Nature 341:758–760
Schiff PB, Fant J, Horwitz SB (1979) Promotion of microtubule assembly in vitro by taxol. Nature 277:665–667
Lee K, Zhang H, Qian DZ, Rey S, Liu JO, Semenza GL (2009) Acriflavine inhibits HIF-1 dimerization, tumor growth, and vascularization. Proc Natl Acad Sci U S A 106:17910–17915
Jung HJ, Shim JS, Lee J, Song YM, Park KC, Choi SH, Kim ND, Yoon JH, Mungai PT, Schumacker PT et al (2010) Terpestacin inhibits tumor angiogenesis by targeting UQCRB of mitochondrial complex III and suppressing hypoxia-induced reactive oxygen species production and cellular oxygen sensing. J Biol Chem 285:11584–11595
Chandel NS, Maltepe E, Goldwasser E, Mathieu CE, Simon MC, Schumacker PT (1998) Mitochondrial reactive oxygen species trigger hypoxia-induced transcription. Proc Natl Acad Sci U S A 95:11715–11720
Kim KH, Park JY, Jung HJ, Kwon HJ (2011) Identification and biological activities of a new antiangiogenic small molecule that suppresses mitochondrial reactive oxygen species. Biochem Biophys Res Commun 404:541–545
Lai TS, Liu Y, Tucker T, Daniel KR, Sane DC, Toone E, Toone E, Burke JR, Strittmatter WJ, Greenberg CS (2008) Identification of chemical inhibitors to human tissue transglutaminase by screening existing drug libraries. Chem Biol 15:969–978
Schulz MM, Reisen F, Zgraggen S, Fischer S, Yuen D, Kang GJ, Kang GJ, Chen L, Schneider G, Detmar M (2012) Phenotype-based high-content chemical library screening identifies statins as inhibitors of in vivo lymphangiogenesis. Proc Natl Acad Sci U S A 109:E2665–E2674
Lin X, David CA, Donnelly JB, Michaelides M, Chandel NS, Huang X, Warrior U, Weinberg F, Tormos KV, Fesik SW et al (2008) A chemical genomics screen highlights the essential role of mitochondria in HIF-1 regulation. Proc Natl Acad Sci U S A 105:174–179
Gohil VM, Sheth SA, Nilsson R, Wojtovich AP, Lee JH, Perocchi F, Chen W, Clish CB, Ayata C, Brookes PS et al (2010) Nutrient-sensitized screening for drugs that shift energy metabolism from mitochondrial respiration to glycolysis. Nat Biotechnol 28:249–255
Yoon J, Koo KH, Choi KY (2011) MEK1/2 inhibitors AS703026 and AZD6244 may be potential therapies for KRAS mutated colorectal cancer that is resistant to EGFR monoclonal antibody therapy. Cancer Res 71:445–453
Huang L, Keyser BM, Tagmose TM, Hansen JB, Taylor JT, Zhuang H, Zhang M, Ragsdale DS, Li M (2004) NNC 55-0396 [(1S,2S)-2-(2-(N-[(3-benzimidazol-2-yl)propyl]-N-methylamino)ethyl)-6-fluoro-1,2,3,4-tetrahydro-1-isopropyl-2-naphtyl cyclopropanecarboxylate dihydrochloride]: a new selective inhibitor of T-type calcium channels. J Pharmacol Exp Ther 309:193–199
Hui AS, Bauer AL, Striet JB, Schnell PO, Czyzyk-Krzeska MF (2006) Calcium signaling stimulates translation of HIF-alpha during hypoxia. FASEB J: Off Publ Fed Am Socr Exp Biol 20:466–475
Yuan G, Nanduri J, Bhasker CR, Semenza GL, Prabhakar NR (2005) Ca2+/calmodulin kinase-dependent activation of hypoxia inducible factor 1 transcriptional activity in cells subjected to intermittent hypoxia. J Biol Chem 280:4321–4328
Del Toro R, Levitsky KL, Lopez-Barneo J, Chiara MD (2003) Induction of T-type calcium channel gene expression by chronic hypoxia. J Biol Chem 278:22316–22324
Guzy RD, Hoyos B, Robin E, Chen H, Liu L, Mansfield KD, Simon MC, Hammerling U, Schumacker PT (2005) Mitochondrial complex III is required for hypoxia-induced ROS production and cellular oxygen sensing. Cell Metab 1:401–408
Vanden Hoek TL, Becker LB, Shao Z, Li C, Schumacker PT (1998) Reactive oxygen species released from mitochondria during brief hypoxia induce preconditioning in cardiomyocytes. J Biol Chem 273:18092–18098
Triantafyllou A, Liakos P, Tsakalof A, Georgatsou E, Simos G, Bonanou S (2006) Cobalt induces hypoxia-inducible factor-1alpha (HIF-1alpha) in HeLa cells by an iron-independent, but ROS-, PI-3 K- and MAPK-dependent mechanism. Free Radic Res 40:847–856
Forsythe JA, Jiang BH, Iyer NV, Agani F, Leung SW, Koos RD, Semenza GL (1996) Activation of vascular endothelial growth factor gene transcription by hypoxia-inducible factor 1. Mol Cell Biol 16:4604–4613
Plate KH, Breier G, Weich HA, Risau W (1992) Vascular endothelial growth-factor is a potential tumor angiogenesis factor in human gliomas invivo. Nature 359:845–848
Semenza GL (2013) Cancer-stromal cell interactions mediated by hypoxia-inducible factors promote angiogenesis, lymphangiogenesis, and metastasis. Oncogene 32:4057–4063
Arnould T, Michiels C, Alexandre I, Remacle J (1992) Effect of hypoxia upon intracellular calcium concentration of human endothelial cells. J Cell Physiol 152:215–221
Seta KA, Yuan Y, Spicer Z, Lu G, Bedard J, Ferguson TK, Pathrose P, Cole-Strauss A, Kaufhold A, Millhorn DE (2004) The role of calcium in hypoxia-induced signal transduction and gene expression. Cell Calcium 36:331–340
Levitsky KL, Lopez-Barneo J (2009) Developmental change of T-type Ca2+ channel expression and its role in rat chromaffin cell responsiveness to acute hypoxia. J Physiol 587:1917–1929
Wan J, Yamamura A, Zimnicka AM, Voiriot G, Smith KA, Tang H, Ayon RJ, Choudhury MS, Ko EA, Wang J et al (2013) Chronic hypoxia selectively enhances L- and T-type voltage-dependent Ca2+ channel activity in pulmonary artery by upregulating Cav1.2 and Cav3.2. Am J Physiol Lung Cell Mol Physiol 305:L154–L164
Yuan G, Nanduri J, Khan S, Semenza GL, Prabhakar NR (2008) Induction of HIF-1alpha expression by intermittent hypoxia: involvement of NADPH oxidase, Ca2+ signaling, prolyl hydroxylases, and mTOR. J Cell Physiol 217:674–685
Oda S, Oda T, Takabuchi S, Nishi K, Wakamatsu T, Tanaka T, Adachi T, Fukuda K, Nohara R, Hirota K (2009) The calcium channel blocker cilnidipine selectively suppresses hypoxia-inducible factor 1 activity in vascular cells. Eur J Pharmacol 606:130–136
Acknowledgments
This study was partly supported by grants from the National Research Foundation of Korea funded by the Korean government (MSIP; 2010-0017984, 2012M3A9D1054520), the Translational Research Center for Protein Function Control, KRF (2009-0083522), the Next-Generation BioGreen 21 Program (No. PJ0079772012), Rural Development Administration, National R&D Program, Ministry of Health &Welfare (0620360-1), and the Brain Korea 21 Plus Project, Republic of Korea.
Conflict of interest
There is no conflict of interest to disclose.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kim, K.H., Kim, D., Park, J.Y. et al. NNC 55-0396, a T-type Ca2+ channel inhibitor, inhibits angiogenesis via suppression of hypoxia-inducible factor-1α signal transduction. J Mol Med 93, 499–509 (2015). https://doi.org/10.1007/s00109-014-1235-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00109-014-1235-1