Abstract
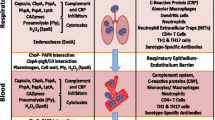
Streptococcus pneumoniae encounters a variety of unique cellular situations during colonization of the nasopharynx or invasion into the lungs, the bloodstream, or the central nervous system. The ligand/receptor pairings that enable this progression of disease appear to be shared by many respiratory pathogens suggesting that a primitive “innate invasion” mechanism may underlie the well-known species-specific mechanisms of pathogenesis. That the acute phase of the innate immune response includes elements to interrupt this path supports this concept. However, it also appears that each cell type or organ responds differently to activation of this innate invasion pathway leaving some organs, such as the lung, intact post-infection but others, such as the brain, largely destroyed. This review posits a concept of innate invasion but cautions that organ-specific responses complicate opportunities for a simple approach to protect from organ damage.



Similar content being viewed by others
References
Orihuela C, Mahdavi J, Thornton J, Mann B, Wooldridge K, Abouseada N, Oldfield N, Self T, Ala’Aldeen D, Tuomanen E (2009) Laminin receptor initiates contact of bacteria with the blood brain barrier. J Clin Invest 119:1638–1646
Luo R, Mann B, Lewis W, Rowe A, Heath R, Stewart M, Hamburger A, Sivakolundu S, Lacy E, Bjorkman P, Tuomanen E, Kriwacki R (2005) Solution structure of choline binding protein A of Streptococcus pneumoniae reveals a novel mode of interaction with its human receptor, plgR. EMBO 24:34–43
Hammerschmidt S, Talay S, Brandtzaeg P, Chhatwal G (1997) SpsA, a novel pneumococcal surface protein with specific binding to secretory immunoglobulin A and secretory component. Mol Microbiol 25:1113–1124
Zhang J-R, Mostov K, Lamm M, Nanno M, Shimida S, Ohwaki M, Tuomanen E (2000) The polymeric immunoglobulin receptor translocates pneumococci across human nasopharyngeal epithelial cells. Cell 102:827–837
Leucht C, Simoneau S, Rey C, Vana K, Rieger R, Ida Lasmezas C, Weiss S (2003) The 37 kDa/67 kDa laminin receptor is required for PrpSc propagation in scrapie-infected neuronal cells. EMBO Rep 4:290–295
Gauczynski S, Peyrin J, Haik S, Leucht C, Hundt C, Rieger R, Krasemann S, Deslys J, Dormont D, Ida Lasmezas C, Weiss S (2001) The 37 kDa/67 kDa laminin receptor acts as the cell-surface receptor for the cellular prion protein. EMBO 20:5863–5875
Cundell D, Gerard N, Gerard C, Idanpaan-Heikkila I, Tuomanen E (1995) Streptococcus pneumoniae anchors to activated eukaryotic cells by the receptor for platelet activating factor. Nature 377:435–438
Swords W, Ketterer M, Shao J, Campbell C, Weiser J, Apicella M (2001) Binding of the non-typeable Haemophilus influenzae lipooligosaccharide to the PAF receptor initiates host cell signalling. Cell Microbiol 3:525–536
Virji M, Saunders J, Sims F, Makepeace K, Maskell D, Ferguson D (1993) Pilus-facilitated adherence of Neisseria meningitidis to human epithelial and endothelial cells: modulation of adherence phenotype occurs concurrently with changes in primary amino acid sequence and the glycosylation status of pilin. Mol Microbiol 10:1013–1028
Weiser J, Pan N, McGowan K, Musher D, Martin A, Richards J (1998) Phosphorylcholine on the lipopolysaccharide of Haemophilus influenzae contributes to persistence in the respiratory tract and sensitivity to serum killing mediated by C-reactive protein. J Exp Med 187:631–640
Cundell D, Weiser J, Shen J, Young A, Tuomanen E (1995) Relationship between colonial morphology and adherence of Streptococcus pneumoniae. Infect Immun 63:757–761
Weiser J, Austrian R, Sreenivasan P, Masure H (1994) Phase variation in pneumococcal opacity: relationship between colonial morphology and nasopharyngeal colonization. Infect Immun 62:2582–2589
Weiser J, Goldberg J, Pan N, Wilson L, Virji M (1998) The phosphorylcholine epitope undergoes phase variation on a 43-kilodalton prpotein in Pseudomonas aeruginosa and on pili of Neisseria. Infect Immun 66:4263–4267
Gmur R, Thurnheer T, Guggenheim B (1999) Dominant cross-reactive antibodies generated during the response to a variety of oral bacterial species detect phosphorylcholine. J Dent Res 78:77–85
Radin J, Orihuela C, Murti G, Guglielmo C, Murray P, Tuomanen E (2005) ß-arrestin 1 determines the traffic pattern of PAFr-mediated endocytosis of Streptococcus pneumoniae. Infect Immun 73:7827–7835
Rijneveld A, Weijer S, Florquin S, Speelman P, Shimizu T, Ishii S, van der Poll T (2004) Improved host defense against pneumococcal pneumonia in platelet-activating factor receptor-deficient mice. J Infect Dis 189:711–716
Pang B, Winn D, Johnson R, Hong W, West-Barnette S, Kock N, Swords W (2008) Lipooligosaccharides containing phosphorylcholine delay pulmonary clearance of nontypeable Haemophilus influenzae. Infect Immun 76:2037–2043
Gehre F, Spisek R, Kharat A, Matthews P, Kukreja A, Anthony R, Dhodapkar M, Vollmer W, Tomasz A (2009) Role of teichoic acid choline moieties in the virulence of Streptococcus pneumoniae. Infect Immun 77:2824–2831
Serino L, Virji M (2000) Phosphorylcholine decoration of lipopolysaccharide differentiates commensal Neisseriae from pathogenic strains: identification of licA-type genes in commensal Neisseriae. Mol Microbiol 36:784–795
Gillespie S, Ainscough S, Dickens A, Lewin J (1996) Phosphorylcholine–containing antigens in bacteria from the mouth and respiratory tract. J Med Microbiol 44:35–40
Barbier M, Oliver A, Rao J, Hanna S, Goldberg J, Alberti S (2008) Novel phosphorylcholine-containing protein of Pseudomonas aeruginosa chronic infection isolates interacts with airway epithelial cells. J Infect Dis 197:465–473
Harper M, Cox A, St Michael F, Parnas H, Wilkie I, Blackall P, Adler B, Boyce J (2007) Decoration of Pasteurella multocida lipopolysaccharide with phosphocholine is important for virulence. J Bacteriol 189:7384–7391
Galvan E, Chen H, Schifferli D (2007) The Psa fimbriae of Yersinia pestis interact with phosphatidylcholine on alveolar epithelial cells and pulmonary surfactant. Infect Immun 75:1272–1279
Gould J, Weiser J (2002) The inhibitory effect of C-reactive protein on bacterial phosphorylcholine platelet-activating factor receptor-mediated adherence is blocked by surfactant. J Infect Dis 186:361–371
Casey R, Newcombe J, McFadden J, Bodman-Smith K (2008) The acute-phase reactant C-reactive protein binds to phosphorylcholine-expressing Neisseria meningitidis and increases uptake by human phagocytes. Infect Immun 76:1298–1304
Goldenberg H, McCool T, Weiser J (2004) Cross-reactivity of human immunoglobulin G2 recognizing phosphorylcholine and evidence for protection against major bacterial pathogens of the human respiratory tract. J Infect Dis 190:1254–1263
Fillon S, Soulis K, Rajasekaran S, Benedict-Hamilton H, Radin J, Orihuela C, El Kasmi K, Murti G, Kaushal D, Gaber M, Weber J, Murray P, Tuomanen E (2006) Platelet activating factor and innate immunity: uptake of Gram positive bacterial cell wall into host cells and cell-specific pathophysiology. J Immunol 177:6182–6191
Braun J, Novak R, Bodmer S, Cleveland J, Tuomanen E (1999) Neuroprotection by a caspase inhibitor in acute bacterial meningitis. Nature Medicine 5:298–302
Braun J, Novak R, Murray P, Eischen C, Susin S, Kroemer G, Halle A, Weber J, Tuomanen E, Cleveland J (2001) Apoptosis inducing factor mediates microglial and neuronal apoptosis caused by pneumococcus. J Infect Dis 184:1300–1309
Arditi M, Mason E, Bradley J (1998) Three year multicenter surveillance of pneumococcal meningitis in children. Pediatrics 102:1087–1097
Nau R, Soto A, Bruck W (1999) Apoptosis of neurons in the dentate gyrus in humans suffereing from bacterial meningitis. J Neuropathol Exp Neurol 58:265–274
Beisswenger C, Coyne C, Shchepetov M, Weiser J (2007) Role of p38 MAP kinase and Transforming Growth Factor ß signaling in transepithelial migration of invasive bacterial pathogens. J Biol Chem 282:28700–28708
Tuomanen E, Liu H, Hengstler B, Zak O, Tomasz A (1985) The induction of meningeal inflammation by components of the pneumococcal cell wall. J Infec Dis 151:859–868
Acknowledgement
This work was supported by NIH grants R01 AI27913 and CA21765 and by the American Lebanese Syrian Associated Charities.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Thornton, J.A., Durick-Eder, K. & Tuomanen, E.I. Pneumococcal pathogenesis: “innate invasion” yet organ-specific damage. J Mol Med 88, 103–107 (2010). https://doi.org/10.1007/s00109-009-0578-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00109-009-0578-5