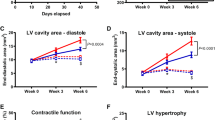
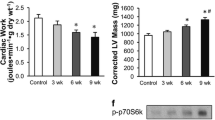
Abstract
Hypertrophic cardiomyopathy (HCM) is associated with cardiac hypertrophy, diastolic dysfunction, and sudden death. Recently, it has been suggested that inefficient energy utilization could be a common molecular pathway of HCM-related mutations. We have previously generated transgenic Sprague–Dawley rats overexpressing a truncated cardiac troponin T (DEL-TNT) molecule, displaying typical features of HCM such as diastolic dysfunction and an increased susceptibility to ventricular arrhythmias. We now studied these rats using 31P magnetic resonance spectroscopy (MRS). MRS demonstrated that cardiac energy metabolism was markedly impaired, as indicated by a decreased phosphocreatine to ATP ratio (−31%, p < 0.05). In addition, we assessed contractility of isolated cardiomyocytes. While DEL-TNT and control cardiomyocytes showed no difference under baseline conditions, DEL-TNT cardiomyocytes selectively exhibited a decrease in fractional shortening by 28% after 1 h in glucose-deprived medium (p < 0.05). Moreover, significant decreases in contraction velocity and relaxation velocity were observed. To identify the underlying molecular pathways, we performed transcriptional profiling using real-time PCR. DEL-TNT hearts exhibited induction of several genes critical for cardiac energy supply, including CD36, CPT-1/-2, and PGC-1α. Finally, DEL-TNT rats and controls were studied by radiotelemetry after being stressed by isoproterenol, revealing a significantly increased frequency of arrhythmias in transgenic animals. In summary, we demonstrate profound energetic alterations in DEL-TNT hearts, supporting the notion that inefficient cellular ATP utilization contributes to the pathogenesis of HCM.





Similar content being viewed by others
References
Ashrafian H, Watkins H (2007) Reviews of translational medicine and genomics in cardiovascular disease: new disease taxonomy and therapeutic implications cardiomyopathies: therapeutics based on molecular phenotype. J Am Coll Cardiol 49:1251–1264
Arad M, Seidman JG, Seidman CE (2002) Phenotypic diversity in hypertrophic cardiomyopathy. Hum Mol Genet 11:2499–2506
Thierfelder L, Watkins H, MacRae C, Lamas R, McKenna W, Vosberg HP, Seidman JG, Seidman CE (1994) Alpha-tropomyosin and cardiac troponin T mutations cause familial hypertrophic cardiomyopathy: a disease of the sarcomere. Cell 77:701–712
Sata M, Ikebe M (1996) Functional analysis of the mutations in the human cardiac beta-myosin that are responsible for familial hypertrophic cardiomyopathy. Implication for the clinical outcome. J Clin Invest 98:2866–2873
Lowey S (2002) Functional consequences of mutations in the myosin heavy chain at sites implicated in familial hypertrophic cardiomyopathy. Trends Cardiovasc Med 12:348–354
Moolman JC, Corfield VA, Posen B, Ngumbela K, Seidman C, Brink PA, Watkins H (1997) Sudden death due to troponin T mutations. J Am Coll Cardiol 29:549–555
Tobacman LS, Nihli M, Butters C, Heller M, Hatch V, Craig R, Lehman W, Homsher E (2002) The troponin tail domain promotes a conformational state of the thin filament that suppresses myosin activity. J Biol Chem 277:27636–27642
Montgomery DE, Tardiff JC, Chandra M (2001) Cardiac troponin T mutations: correlation between the type of mutation and the nature of myofilament dysfunction in transgenic mice. J Physiol 536:583–592
Ashrafian H, Redwood C, Blair E, Watkins H (2003) Hypertrophic cardiomyopathy: a paradigm for myocardial energy depletion. Trends Genet 19:263–268
Crilley JG, Boehm EA, Blair E, Rajagopalan B, Blamire AM, Styles P, McKenna WJ, Ostman-Smith I, Clarke K, Watkins H (2003) Hypertrophic cardiomyopathy due to sarcomeric gene mutations is characterized by impaired energy metabolism irrespective of the degree of hypertrophy. J Am Coll Cardiol 41:1776–1782
Tanaka T, Sohmiya K, Kawamura K (1997) Is CD36 deficiency an etiology of hereditary hypertrophic cardiomyopathy? J Mol Cell Cardiol 29:121–127
Merante F, Tein I, Benson L, Robinson BH (1994) Maternally inherited hypertrophic cardiomyopathy due to a novel T-to-C transition at nucleotide 9997 in the mitochondrial tRNA(glycine) gene. Am J Hum Genet 55:437–446
Frey N, Franz WM, Gloeckner K, Degenhardt M, Muller M, Muller O, Merz H, Katus HA (2000) Transgenic rat hearts expressing a human cardiac troponin T deletion reveal diastolic dysfunction and ventricular arrhythmias. Cardiovasc Res 47:254–264
Frey N, Brixius K, Schwinger RH, Benis T, Karpowski A, Lorenzen HP, Luedde M, Katus HA, Franz WM (2006) Alterations of tension-dependent ATP utilization in a transgenic rat model of hypertrophic cardiomyopathy. J Biol Chem 281:29575–29582
Flogel U, Jacoby C, Godecke A, Schrader J (2007) In vivo 2D mapping of impaired murine cardiac energetics in NO-induced heart failure. Magn Reson Med 57:50–58
Winer J, Jung CK, Shackel I, Williams PM (1999) Development and validation of real-time quantitative reverse transcriptase-polymerase chain reaction for monitoring gene expression in cardiac myocytes in vitro. Anal Biochem 270:41–49
Tardiff JC, Factor SM, Tompkins BD, Hewett TE, Palmer BM, Moore RL, Schwartz S, Robbins J, Leinwand LA (1998) A truncated cardiac troponin T molecule in transgenic mice suggests multiple cellular mechanisms for familial hypertrophic cardiomyopathy. J Clin Invest 101:2800–2811
Vega RB, Huss JM, Kelly DP (2000) The coactivator PGC-1 cooperates with peroxisome proliferator-activated receptor alpha in transcriptional control of nuclear genes encoding mitochondrial fatty acid oxidation enzymes. Mol Cell Biol 20:1868–1876
Czubryt MP, McAnally J, Fishman GI, Olson EN (2003) Regulation of peroxisome proliferator-activated receptor gamma coactivator 1 alpha (PGC-1 alpha) and mitochondrial function by MEF2 and HDAC5. Proc Natl Acad Sci USA 100:1711–1716
Hasenfuss G, Mulieri LA, Allen PD, Just H, Alpert NR (1996) Influence of isoproterenol and ouabain on excitation–contraction coupling, cross-bridge function, and energetics in failing human myocardium. Circulation 94:3155–3160
Redwood CS, Moolman-Smook JC, Watkins H (1999) Properties of mutant contractile proteins that cause hypertrophic cardiomyopathy. Cardiovasc Res 44:20–36
Sweeney HL, Feng HS, Yang Z, Watkins H (1998) Functional analyses of troponin T mutations that cause hypertrophic cardiomyopathy: insights into disease pathogenesis and troponin function. Proc Natl Acad Sci USA 95:14406–14410
Spindler M, Saupe KW, Christe ME, Sweeney HL, Seidman CE, Seidman JG, Ingwall JS (1998) Diastolic dysfunction and altered energetics in the alphaMHC403/+ mouse model of familial hypertrophic cardiomyopathy. J Clin Invest 101:1775–1783
Javadpour MM, Tardiff JC, Pinz I, Ingwall JS (2003) Decreased energetics in murine hearts bearing the R92Q mutation in cardiac troponin T. J Clin Invest 112:768–775
Sakata K, Ino H, Fujino N, Nagata M, Uchiyama K, Hayashi K, Konno T, Inoue M, Kato H, Sakamoto Y, Tsubokawa T, Yamagishi M (2008) Exercise-induced systolic dysfunction in patients with non-obstructive hypertrophic cardiomyopathy and mutations in the cardiac troponin genes. Heart 94:1282–1287
Shimizu M, Ino H, Yamaguchi M, Terai H, Uchiyama K, Inoue M, Ikeda M, Kawashima A, Mabuchi H (2003) Autopsy findings in siblings with hypertrophic cardiomyopathy caused by Arg92Trp mutation in the cardiac troponin T gene showing dilated cardiomyopathy-like features. Clin Cardiol 26:536–539
Lehman JJ, Barger PM, Kovacs A, Saffitz JE, Medeiros DM, Kelly DP (2000) Peroxisome proliferator-activated receptor gamma coactivator-1 promotes cardiac mitochondrial biogenesis. J Clin Invest 106:847–856
Varnava AM, Elliott PM, Baboonian C, Davison F, Davies MJ, McKenna WJ (2001) Hypertrophic cardiomyopathy: histopathological features of sudden death in cardiac troponin T disease. Circulation 104:1380–1384
Maron BJ, Fananapazir L (1992) Sudden cardiac death in hypertrophic cardiomyopathy. Circulation 85:I57–I63
Spirito P, Bellone P, Harris KM, Bernabo P, Bruzzi P, Maron BJ (2000) Magnitude of left ventricular hypertrophy and risk of sudden death in hypertrophic cardiomyopathy. N Engl J Med 342:1778–1785
Marian AJ, Roberts R (2001) The molecular genetic basis for hypertrophic cardiomyopathy. J Mol Cell Cardiol 33:655–670
Wolf CM, Moskowitz IP, Arno S, Branco DM, Semsarian C, Bernstein SA, Peterson M, Maida M, Morley GE, Fishman G, Berul CI, Seidman CE, Seidman JG (2005) Somatic events modify hypertrophic cardiomyopathy pathology and link hypertrophy to arrhythmia. Proc Natl Acad Sci USA 102:18123–18128
Knollmann BC, Kirchhof P, Sirenko SG, Degen H, Greene AE, Schober T, Mackow JC, Fabritz L, Potter JD, Morad M (2003) Familial hypertrophic cardiomyopathy-linked mutant troponin T causes stress-induced ventricular tachycardia and Ca2+-dependent action potential remodeling. Circ Res 92:428–436
Chandra M, Tschirgi ML, Tardiff JC (2005) Increase in tension-dependent ATP consumption induced by cardiac troponin T mutation. Am J Physiol Heart Circ Physiol 289:H2112–H2119
Acknowledgements
The authors thank Ulrike Oehl and Jutta Krebs for their excellent technical support.
Conflict of Interest
None declared
Funding
N.F. was supported by the Bundesministerium für Bildung und Forschung, Germany (Nationales Genomforschungsnetz: NGFN2 and NGFNplus: 01650836), U.F. and J.S. were supported by the SFB 612 “Molekulare Analyse kardiovaskulärer Funktionen and Funktionsstörungen”, Teilprojekt Z2, and the DFG project SCHR154/9.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Material
(DOC 291 kb)
Supplemental Figure
Stimulation of WT and DEL-TNT cardiomyocytes with 10 nm isoproterenol [ISO] led to a significant increase of fractional shortening in WT and DEL-TNT cardiomyocytes. After 1 h of glucose depletion, DEL-TNT cells that were stimulated with isoproterenol revealed a significant decrease in fractional shortening (−47% ± 6.2%) that was comparable to the decrease of FS in DEL-TNT CMs that were not stimulated with ISO (−46% ± 9.8%). In contrast, WT CMs revealed no significant decrease in FS after 1-h glucose depletion with or without ISO stimulation. Data are mean values ± SEM. *p < 0.05, †p < 0.01, ‡p < 0.001 (DOC 20.0 kb)
Rights and permissions
About this article
Cite this article
Luedde, M., Flögel, U., Knorr, M. et al. Decreased contractility due to energy deprivation in a transgenic rat model of hypertrophic cardiomyopathy. J Mol Med 87, 411–422 (2009). https://doi.org/10.1007/s00109-008-0436-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00109-008-0436-x