Abstract
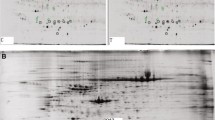
The ovary plays a central role in oogenesis and gonadal hormone secretion. Proteomic analysis is a valuable approach for gaining an increased understanding of the molecular nature of the ovary. In this work, two-dimensional electrophoresis for protein separation followed by matrix-assisted laser desorption/ionization mass spectrometry and database searches, identified 231 protein spots corresponding to 138 individual proteins that were found in gels representing both the follicular and luteal phases. The data were used to construct a database online (http://reprod.njmu.edu.cn/2d). The identified proteins were functionally classified into seven groups: (1) cell signaling/communication, (2) cell division, (3) gene/protein expression, (4) metabolism, (5) cell structure and motility, (6) cell/organism defense, and (7) unclassified. Among the proteins identified, 47% had not been previously reported in the human ovary. In addition, a number of disease-related proteins were identified in this protein map, including some cancer- and polycystic ovarian syndrome-related proteins. Two proteins with phosphorylation were verified by Western blot analysis. Comparison of protein abundance between follicular and luteal stages produced seven protein spots that had been identified in our database. This study provides a preliminary reference map of normal human ovary that will form a basis for comparative studies on normal and pathological conditions of the human ovary and may serve as a potential tool for clinical diagnosis, therapeutics, and prognosis.







Similar content being viewed by others
Abbreviations
- PCOS:
-
polycystic ovarian syndrome
- 2-D PAGE:
-
two-dimensional polyacrylamide gel electrophoresis
- CHAPS:
-
3-[(3-cholamidopropyl)-dimethylammonio]-1-propane sulfonate
- IPG:
-
immobilized pH gradient
- SDS:
-
sodium dodecyl sulphate
- APS:
-
ammonium persulfate
- TEMED:
-
N,N,N′,N′-tetramethylethylenediamine
- ACN:
-
acetonitrile
- TFA:
-
trifluoroacetic acid
- DTT:
-
dithiothreitol
- hnRNP A2/B1:
-
heterogeneous nuclear ribonucleoproteins A2/B1
- IEF:
-
Isoelectric focusing
- MALDI-TOF:
-
matrix-assisted laser desorption/ionization time-of-flight
- pI:
-
isoelectric points
- MWs:
-
molecular weights
- ANX:
-
annexin
- HCC:
-
hepatocellular carcinoma
- VDAC:
-
voltage-dependent anion-selective channel
- BAK:
-
BCL2 homologous antagonist/killer
References
Britt KL, Findlay JK (2002) Estrogen actions in the ovary revisited. Endocrinology 175:269–276
Pinter JH, Deep C, Park-Sarge OK (1996) Progesterone receptors: expression and regulation in the mammalian ovary. Clin Obstet Gynecol 39:424–435
Berek JS, Martinez-Maza O (1994) Molecular and biologic factors in the pathogenesis of ovarian cancer. J Reprod Med 39:241–248
Goshen R, Weissman A, Shoham Z (1998) Epithelial ovarian cancer, infertility and induction of ovulation: possible pathogenesis and updated concepts. Baillieres Clin Obstet Gynaecol 12:581–591
Barnes R, Rosenfield RL (1989) The polycystic ovary syndrome: pathogenesis and treatment. Ann Intern Med 110:386–399
Marx TL, Mehta AE (2003) Polycystic ovary syndrome: pathogenesis and treatment over the short and long term. Cleve Clin J Med 70:31–33, 36–41, 45
Diao FY, Xu M, Li JM, Xu ZY, Lin M, Wang LR, Zhou YD, Zhou ZM, Liu JY, Sha JH (2004) The molecular characteristics of polycystic ovary syndrome (PCOS) ovary defined by human ovary cDNA microarray. J Mol Endocrinol 33:59–72
Wasinger VC, Cordwell SJ, Cerpa-Poljak A, Yan JX, Gooley AA, Wilkins MR, Duncan MW, Harris R, Williams KL, Humphery-Smith I (1995) Progress with gene-product mapping of the mollicutes: mycoplasma genitalium. Electrophoresis 16:1090–1094
Pennington SR, Wilkins MR, Hochwtrasser DF, Dunn MJ (1997) Proteome analysis: from protein characterization to biological function. Trends Cell Biol 7:168–173
Jungblut PR, Zimny-Arndt U, Zeindl-Eberhart E, Stulik J, Koupilova K, Pleissner KP, Otto A, Muller EC, Sokolowska-Kohler W, Grabher G, Stoffler G (1999) Proteomics in human disease: cancer, heart and infectious diseases. Electrophoresis 20:2100–2110
Sarioglu H, Lottspeich F, Walk T, Jung G, Eckerskorn C (2000) Deamidation as a widespread phenomenon in two-dimensional polyacrylamide gel electrophoresis of human blood plasma proteins. Electrophoresis 21:2209–2218
Thiede B, Siejak F, Dimmler C, Jungblut PR, Rudel T (2000) A two-dimensional electrophoresis database of a human Jurkat T-cell line. Electrophoresis 21:2713–2720
Leak LV, Liotta LA, Krutzsch H, Jones M, Fusaro VA, Ross SJ, Zhao YM, Petricoin EF III (2004) Proteomic analysis of lymph. Proteomics 4:753–765
Garcia A, Prabhakar S, Brock CJ, Pearce AC, Dwek RA, Watson SP, Hebestreit HF, Zitzmann N (2004) Extensive analysis of the human platelet proteome by two-dimensional gel electrophoresis and mass spectrometry. Proteomics 4:656–668
Hoogland C, Sanchez JC, Walther D, Baujard V, Baujard O, Tonella L, Hochstrasser DF, Appel RD (1999) Two-dimensional electrophoresis resources available from ExPASy. Electrophoresis 20:3568–3571
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Shevchenko A, Wilm M, Vorm O, Mann M (1996) Mass spectrometric sequencing of proteins from silver-stained polyacrylamide gels. Anal Chem 68:850–858
Neuhoff V, Arold N, Taube D, Ehrhardt W (1988) Improved staining of proteins in polyacrylamide gels including isoelectric focusing gels with clear background at nanogram sensitivity using Coomassie Brilliant Blue G-250 and R-250. Electrophoresis 9:255–262
Com E, Evrard B, Roepstorff P, Aubry F, Pineau C (2003) New insights into the rat spermatogonial proteome. Mol Cell Proteomics 2:248–261
Adams MD, Kerlavage AR, Fleischmann RD, Fuldner RA, Bult CJ, Lee NH, Kirkness EF, Weinstock KG, Gocayne JD, White O et al (1995) Initial assessment of human gene diversity and expression patterns based upon 83 million nucleotides of cDNA sequence. Nature 377(6547 Suppl):3–174
Roche JF (1996) Control and regulation of folliculogenesis—a symposium in perspective. Rev Reprod 1:19–27
Ono S, Yoshihiko Y, Yamashiro S, Matsudaira PT, Gnarra JR, Obinata T, Matsumura F (1997) Identification of an actin binding region and a protein kinase C phosphorylation site on human fascin. J Biol Chem 272:2527–2533
Hwang JL, Menon KMJ (1985) Binding of apolipoprotein A-I and A-I1 after recombination with phospholipid vesicles to the high-density lipoprotein receptor of luteinized rat ovary. J Biol Chem 260:5660–5668
Burgoyne RD, Geisow MJ (1989) The annexin family of calcium-binding proteins. Cell Calcium 10:1–10
Tsao FH, Chen X, Chen X, Ts’ao CH (1995) Annexin I in female rabbit reproductive organs: varying levels in relation to maturity and pregnancy. Lipids 30:507–511
Uittenbogaard A, Everson WV, Matveev SV, Smart EJ (2002) Cholesteryl ester is transported from caveolae to internal membranes as part of a caveolin-annexin II lipid-protein complex. J Biol Chem 277:4295–4931
Oliferenko S, Paiha K, Harder T, Gerke V, Schwärzler C, Schwarz H, Beug H, Günthert U, Huber LA (1999) Analysis of CD44-containing lipid rafts: recruitment of annexin II and stabilization by the actin cytoskeleton. J Cell Biol 146:843–854
Maillet G, Benhaim A, Mittre H, Feral C (2003) Involvement of theca cells and steroids in the regulation of granulosa cell apoptosis in rabbit preovulatory follicles. Reproduction 125:709–716
Kawaminami M, Shibata Y, Yaji A, Kurusu S, Hashimoto I (2003) Prolactin inhibits annexin 5 expression and apoptosis in the corpus luteum of pseudopregnant rats: involvement of local gonadotropin-releasing hormone. Endocrinology 144:3625–3631
Maillet G, Breard E, Benhaim A, Leymarie P, Feral C (2002) Hormonal regulation of apoptosis in rabbit granulosa cells in vitro: evaluation by flow cytometric detection of plasma membrane phosphatidylserine externalization. Reproduction 123:243–251
Van den Brule F, Califice S, Garnier F, Fernandez PL (2003) Galectin-1 accumulation in the ovary carcinoma peritumoral stroma is induced by ovary carcinoma cells and affects both cancer cell proliferation and adhesion to laminin-1 and fibronectin. Lab Invest 83:377–386
Thompson WE, Asselin E, Branch A, Stiles JK, Sutovsky P, Lai L, Im GS, Prather RS, Isom SC, Rucker E III, Tsang BK (2004) Regulation of prohibitin expression during follicular development and atresia in the mammalian ovary. Biol Reprod 71:282–290
Williams K, Chubb C, Huberman E, Giometti CS (1998) Analysis of differential protein expression in normal and neoplastic human breast epithelial cell lines. Electroporesis 19:333–343
Asamoto M, Cohen SM (1994) Prohibition gene is overexpressed but not mutated in rat bladder carcinomas and cell lines. Cancer Lett 83:201–207
Cliby M, Sarkar G, Ritland SR, Hartmann L, Podratz KC, Jenkins RB (1993) Absence of prohibitin gene mutations in human epithelial ovarian tumors. Gynecol Oncol 50:34–37
Seow TK, Ong SE, Liang RC, Ren EC, Chan L, Ou K, Chung MC (2000) Two-dimensional electrophoresis map of the human hepatocellular carcinoma cell line, HCC-M, and identification of the separated proteins by mass spectrometry. Electrophoresis 21:1787–1813
Masaki T, Tokuda M, Ohnishi M, Watanabe S, Fujimra T, Miyamoto K, Itano T, Matsui H, Arima K, Shirai M, Maeba T, Sogawa K, Konishi R, Taniguchi K, Hatanaka Y, Hatase O, Nishiokai M (1996) Enhanced expression of the protein kinase substrate annexin I in human hepatocellular carcinoma. Hepatology 24:72–81
Puisieux A, Ji J, Ozturk M (1996) Annexin II up-regulates cellular levels of p11 protein by a post-translational mechanism. Biochem J 313:51–55
Shibata S, Sato H, Ota H, Karube A, Takahashi O, Tanaka T (1997) Involvement of annexin V in antiproliferative effects of gonadotropin-releasing hormone agonists on human endometrial cancer cell line. Gynecol Oncol 66:217–221
Karube A, Shidara Y, Hayasaka K, Maki M, Tanaka T (1995) Suppression of calphobindin I (CPB I) production in carcinoma of uterine cervix and endometrium. Gynecol Oncol 58:295–300
Lawen A, Ly JD, Lane DJR, Zarschler K, Messina A, Pinto VD (2005) Voltage-dependent anion-selective channel 1 (VDAC1)—a mitochondrial protein, rediscovered as a novel enzyme in the plasma membrane. Int J Biochem Cell Biol 37:277–282
Cheng EHY, Sheiko TV, Fisher JK, Craigen WJ, Korsmeyer SJ (2003) VDAC2 inhibits BAK activation and mitochondrial apoptosis. Science 301:513–517
Kamma H, Horiguchi H, Wan L, Matsui M, Fujiwara M, Fujimoto M, Yazawa T, Dreyfuss G (1999) Molecular characterization of the hnRNP A2/B1 proteins: tissue-specific expression and novel isoforms. Exp Cell Res 246:399–411
Acknowledgements
This work was supported by the National Natural Science Foundation of China (30170356).
Author information
Authors and Affiliations
Corresponding author
Additional information
L. Wang and Y.-F. Zhu contributed equally to this work
Electronic Supplementary Material
Rights and permissions
About this article
Cite this article
Wang, L., Zhu, YF., Guo, XJ. et al. A two-dimensional electrophoresis reference map of human ovary. J Mol Med 83, 812–821 (2005). https://doi.org/10.1007/s00109-005-0676-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00109-005-0676-y