Abstract
Introduction
The wound healing properties of platelet-rich plasma (PRP) gel have been documented in many studies. PRP gel has also become a promising agent for treating surgical site infections. In this study, we investigated the antibacterial activity and wound healing effectiveness of PRP in an animal model of Methicillin-resistant Staphylococcus aureus subsp. aureus (MRSA N315)-contaminated superficial soft tissue wounds.
Materials and methods
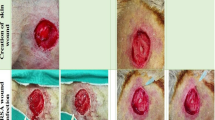
Subcutaneous wounds in Wistar Albino male rats were created by making two cm midline incisions followed by inoculation of microorganisms. Study groups comprised of Sham (no treatment), PRP alone, MRSA alone, MRSA + PRP, MRSA + Vancomycin, and MRSA + Vancomycin + PRP groups. We inoculated 0.1 mL (3 × 108 CFU/mL) of MRSA in contaminated groups. After 8 days, all rats were killed, wounds were excised and subjected to histopathologic examination, and MRSA counts were determined.
Results
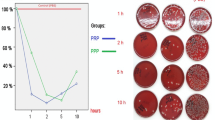
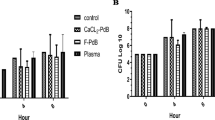
MRSA counts in MRSA, MRSA + PRP, MRSA + Vancomycin and MRSA + Vancomycin + PRP groups were 5.1 × 106 (SD ± 0.4) CFU/mL, 4.3 × 106 (SD ± 0.7) CFU/mL, 2.3 × 106 (SD ± 0.3) CFU/mL, 1.1 × 106 (SD ± 0.4) CFU/mL, respectively. The inflammation scores of MRSA + PRP, MRSA + Vancomycin, and MRSA + Vancomycin + PRP groups were significantly lower than the MRSA group. MRSA + Vancomycin + PRP group inflammation score was significantly lower than the MRSA + PRP group.
Discussion
All treatment groups were effective in wound healing and decreasing the MRSA counts. MRSA + PRP combined created identical inflammation scores to the PRP group. More in vivo studies are required to corroborate these findings.



Similar content being viewed by others
References
Sganga G, Tascini C, Sozio E, Carlini M, Chirletti P, Cortese F, et al. Focus on the prophylaxis, epidemiology and therapy of methicillin-resistant Staphylococcus aureus surgical site infections and a position paper on associated risk factors: the perspective of an Italian group of surgeons. World J Emerg Surg. 2016;11:26.
Martone WJ, Nichols RL. Recognition, prevention, surveillance, and management of surgical site infections: introduction to the problem and symposium overview. Clin Infect Dis. 2001;33(Suppl 2):S67-8.
Noskin GA, Rubin RJ, Schentag JJ, Kluytmans J, Hedblom EC, Smulders M, et al. The burden of Staphylococcus aureus infections on hospitals in the United States: an analysis of the 2000 and 2001 Nationwide Inpatient Sample Database. Arch Intern Med. 2005;165(15):1756–61.
Engemann JJ, Carmeli Y, Cosgrove SE, Fowler VG, Bronstein MZ, Trivette SL, et al. Adverse clinical and economic outcomes attributable to methicillin resistance among patients with Staphylococcus aureus surgical site infection. Clin Infect Dis. 2003;36(5):592–8.
Motta GJ, Milne CT, Corbett LQ. Impact of antimicrobial gauze on bacterial colonies in wounds that require packing. Ostomy Wound Manage. 2004;50(8):48–62.
Amable PR, Carias RB, Teixeira MV, da Cruz Pacheco I, Correa do Amaral RJ, Granjeiro JM, et al. Platelet-rich plasma preparation for regenerative medicine: optimization and quantification of cytokines and growth factors. Stem Cell Res Ther. 2013;4(3):67.
Driver VR, Hanft J, Fylling CP, Beriou JM. Autologel Diabetic Foot Ulcer Study G. A prospective, randomized, controlled trial of autologous platelet-rich plasma gel for the treatment of diabetic foot ulcers. Ostomy Wound Manage. 2006;52(6):68–70 (2, 4 passim).
Lacci KM, Dardik A. Platelet-rich plasma: support for its use in wound healing. Yale J Biol Med. 2010;83(1):1–9.
Sun S, Wang C, Chen D, Cen S, Lv X, Wen X, et al. Combating superbug without antibiotic on a postamputation wound in a patient with diabetic foot. Int J Low Extrem Wounds. 2016;15(1):74 – 7.
Fallon MT, Shafer W, Jacob E. Use of cefazolin microspheres to treat localized methicillin-resistant Staphylococcus aureus infections in rats. J Surg Res. 1999;86(1):97–102.
Li H, Hamza T, Tidwell JE, Clovis N, Li B. Unique antimicrobial effects of platelet-rich plasma and its efficacy as a prophylaxis to prevent implant-associated spinal infection. Adv Healthc Mater. 2013;2(9):1277–84.
Watkins RR, Lemonovich TL, File TM. Jr. An evidence-based review of linezolid for the treatment of methicillin-resistant Staphylococcus aureus (MRSA): place in therapy. Core Evid. 2012;7:131–43.
Jacob JT, DiazGranados CA. High vancomycin minimum inhibitory concentration and clinical outcomes in adults with methicillin-resistant Staphylococcus aureus infections: a meta-analysis. Int J Infect Dis. 2013;17(2):e93–e100.
Yeaman MR. Platelets: at the nexus of antimicrobial defence. Nat Rev Microbiol. 2014;12(6):426–37.
Yeaman MR. Platelets in defense against bacterial pathogens. Cell Mol Life Sci. 2010;67(4):525–44.
Trier DA, Gank KD, Kupferwasser D, Yount NY, French WJ, Michelson AD, et al. Platelet antistaphylococcal responses occur through P2 × 1 and P2Y12 receptor-induced activation and kinocidin release. Infect Immun. 2008;76(12):5706–13.
VanEperen AS, Segreti J. Empirical therapy in methicillin-resistant staphylococcus aureus infections: an up-to-date approach. J Infect Chemother. 2016;22(6):351–9.
Burnouf T, Chou ML, Wu YW, Su CY, Lee LW. Antimicrobial activity of platelet (PLT)-poor plasma, PLT-rich plasma, PLT gel, and solvent/detergent-treated PLT lysate biomaterials against wound bacteria. Transfusion. 2013;53(1):138 – 46.
Mehta S, Watson JT. Platelet rich concentrate: basic science and current clinical applications. J Orthop Trauma. 2008;22(6):432–8.
Landesberg R, Roy M, Glickman RS. Quantification of growth factor levels using a simplified method of platelet-rich plasma gel preparation. J Oral Maxillofac Surg. 2000;58(3):297–300 (discussion-1).
Robson M. Integrating the results of phase IV (postmarketing) clinical trial with four previous trials reinforces the position that Regranex (becaplermin) gel 0.01% is an effective adjunct to the treatment of diabetic foot ulcers. J Appl Res. 2005(5):35–45.
Clawson CC, White JG. Platelet interaction with bacteria. II. Fate of the bacteria. Am J Pathol. 1971;65(2):381 – 97.
Li GY, Yin JM, Ding H, Jia WT, Zhang CQ. Efficacy of leukocyte- and platelet-rich plasma gel (L-PRP gel) in treating osteomyelitis in a rabbit model. J Orthop Res. 2013;31(6):949 – 56.
Lian Z, Yin X, Li H, Jia L, He X, Yan Y, et al. Synergistic effect of bone marrow-derived mesenchymal stem cells and platelet-rich plasma in streptozotocin-induced diabetic rats. Ann Dermatol. 2014;26(1):1–10.
Sakoulas G, Moellering RC, Jr. Increasing antibiotic resistance among methicillin-resistant Staphylococcus aureus strains. Clin Infect Dis. 2008;46(Suppl 5):S360–7.
Bielecki TM, Gazdzik TS, Arendt J, Szczepanski T, Krol W, Wielkoszynski T. Antibacterial effect of autologous platelet gel enriched with growth factors and other active substances: an in vitro study. J Bone Joint Surg Br. 2007;89(3):417–20.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Riza Aytac Cetinkaya, Soner Yilmaz, Aytekin Ünlü, Patrizio Petrone, Corrado P. Marini, Enes Karabulut, Murat Urkan, Erkan Kaya, Kubilay Karabacak, Metin Uyanik, Ibrahim Eker, Abdullah Kilic, Armağan Gunal declare that they have not conflict of interest.
Informed consent
This research involved animals as subjects of the study. The study design for animal procedures was approved by the Ethics Committee of Gulhane Military Medical Academy.
Rights and permissions
About this article
Cite this article
Cetinkaya, R.A., Yilmaz, S., Ünlü, A. et al. The efficacy of platelet-rich plasma gel in MRSA-related surgical wound infection treatment: an experimental study in an animal model. Eur J Trauma Emerg Surg 44, 859–867 (2018). https://doi.org/10.1007/s00068-017-0852-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00068-017-0852-0