Abstract
Purpose
Total body irradiation (TBI) is a common part of the myelo- and immuno-ablative conditioning regimen prior to an allogeneic hematopoietic stem cell transplantation (allo-HSCT). Due to concerns regarding acute and long-term complications, there is currently a decline in otherwise successfully established TBI-based conditioning regimens. Here we present an analysis of patient and treatment data with focus on survival and long-term toxicity.
Methods
Patients with hematologic diseases who received TBI as part of their conditioning regimen prior to allo-HSCT at Frankfurt University Hospital between 1997 and 2015 were identified and retrospectively analyzed.
Results
In all, 285 patients with a median age of 45 years were identified. Median radiotherapy dose applied was 10.5 Gy. Overall survival at 1, 2, 5, and 10 years was 72.6, 64.6, 54.4, and 51.6%, respectively. Median follow-up of patients alive was 102 months. The cumulative incidence of secondary malignancies was 12.3% (n = 35), with hematologic malignancies and skin cancer predominating. A TBI dose ≥ 8 Gy resulted in significantly improved event-free (p = 0.030) and overall survival (p = 0.025), whereas a total dose ≤ 8 Gy and acute myeloid leukemia (AML) diagnosis were associated with significantly increased rates of secondary malignancies (p = 0.003, p = 0.048) in univariate analysis. No significant correlation was observed between impaired renal or pulmonary function and TBI dose.
Conclusion
TBI remains an effective and well-established treatment, associated with distinct late-toxicity. However, in the present study we cannot confirm a dose–response relationship in intermediate dose ranges. Survival, occurrence of secondary malignancies, and late toxicities appear to be subject to substantial confounding in this context.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Total body irradiation (TBI) is a common and effective part of the myelo- and immuno-ablative conditioning regimen prior to an allogeneic hematopoietic stem cell transplantation (allo-HSCT) for patients with malignant and nonmalignant hematologic diseases [1]. Since its introduction by Nobel Prize Laureate E. D. Thomas in the late 1950s [2, 3], TBI concepts have evolved [4]. Early treatment regimens combined a lethal dose of TBI with or without cyclophosphamide (Cy) to eradicate cancer cells in patients with acute myeloid leukemia (AML) [5]. Although, improvements regarding technical aspects and fractionation have reduced acute and late toxicities [6, 7], TBI is associated with immediate and long-term posttransplant complications which can impair both quality of life and outcome [8,9,10]. In general, complications after allo-HSCT are multifactorial, but there is a broad spectrum of particularly radiation-related side effects including cataract, endocrine and gonadal insufficiency, renal malfunction, lung fibrosis, cardiomyopathy, veno-occlusive disease (VOD) and others [11, 12]. Moreover, the development of secondary malignancies is a serious long-term toxicity, caused by extensive radiation and chemotherapy as well as the profound immunosuppression after allo-HSCT [13,14,15,16]. As a consequence, alternative conditioning protocols were pursued, and reduced-intensity and chemotherapy-based regimens were implemented [17,18,19]. Further improvements in donor selection and high-resolution human leukocyte antigen (HLA) matching, as well as management of infections posttransplant, prevention and treatment of graft-versus-host disease (GVHD) have ameliorated treatment-related mortality and morbidity significantly [20, 21]. Accordingly, chemotherapy-based and reduced-intensity conditioning (RIC) regimens have become increasingly well accepted and are substituting otherwise successfully established TBI-based treatment regimens due to general concerns regarding tolerability and toxicity [22, 23].
These developments resulted in a constant decline in the application of TBI as a first-line treatment modality. Nevertheless, contradictory results from previous analyses leave the question of the optimal conditioning regimen unanswered, while an increasing number of patients qualifying for an intensified therapy protocol after relapse [24,25,26]. Endpoints of this study are the long-term survival and the incidence of late toxicity, including secondary malignancies, after TBI-based conditioning regimens of various intensities prior to allo-HSCT in a single-center cohort of 285 patients.
Patients and methods
Patients—inclusion criteria
We performed a retrospective analysis of patients with hematologic diseases, who underwent a TBI as part of the myelo- and immune-ablative conditioning regimen prior to allo-HSCT at the University Hospital Frankfurt between 1997 and December 2015. Patients had to be 18 years or older at the time of irradiation and received at least one fraction of TBI. Adolescents (≥ 16 years) treated in analogy to protocols for adult patients were also included. The choice of conditioning regimen was made by the treating physicians according to the respective standard of care, within a clinical trial, or after an interdisciplinary tumor conference. Data on disease control and toxicity were retrieved from the patient files. In addition, patients alive were also contacted and asked to complete a specific questionnaire including questions about general health status, comorbidities and any therapy-associated malignancies according to organ systems. The study was approved by the institutional review board (approval number 221/16).
Radiotherapy—conditioning chemotherapy regimens
All radiotherapy series were applied with linear accelerators, using exclusively 6 MV energy photons and implementing a motorized patient translational couch (Barry-Liege®, Jochen Barry GmbH, Essen, Germany) with variable, preselectable transport speed moving the patient through an open radiation field. The radiation field measures 15 cm in length and 40 cm in width at the isocenter. TBI was performed in two daily fractions of 1.8 Gy (1997–2003) or 2 Gy (2003 until today), administered at least 6 h apart. The patients were irradiated alternating in prone and supine position for each Gray (Gy) of dose applied. A tissue-equivalent bolus in the neck region is used to reduce the risk of overdose due to a smaller diameter. In addition, a Plexiglas® (Röhm GmbH, Darmstadt, Germany) (polymethylmethacrylate glass) bolus was used to cover the entire field below the eyes to increase the skin dose. In vivo dosimetry with semiconducting diodes was performed for dose verification. Six semiconducting diodes were placed under the eye, on the neck behind the bolus, behind the ventral lung block, in the dorsal lung region, near the umbilicus (i.e., the ventral reference point) and its dorsal counterpart (i.e., the dorsal reference point). The detailed procedure was previously described by Ramm et al. in 2008 [27]. Customized, partial lung shields were used for prescribed doses > 10 Gy.
For patients with acute leukemia, treatment regimens typically included a remission induction chemotherapy followed by a conditioning regimen in preparation for allo-HSCT. Standard remission induction chemotherapy for patients with acute myeloid leukemia (AML) consisted of one or two courses of cytarabine and anthracycline (7 + 3) [28]. After induction chemotherapy, allo-HSCT was recommended for (1) suitable patients in first complete remission with intermediate- or high-risk disease determined by cytogenetic or molecular genetic abnormalities and (2) for patients with relapsed disease [29, 30]. Patients with acute lymphoblastic leukemia (ALL) were treated within or according to the respective protocols of the GMALL study group and typically transplanted after the first consolidation cycle [31].
After completion of induction chemotherapy, the conditioning regimen was evaluated. These are fixed combinations adapted to the patient and risk factors with a characteristic profile of adverse events and toxicities. For patients with AML or ALL in complete remission up to the age of 40–50 years, the most frequently used myeloablative conditioning (MAC) regimen consisted of 12 Gy TBI in combination with cyclophosphamide or etoposide. Patients above the age of 40 or with comorbidities were eligible for a reduced intensity conditioning (RIC) regimen of 8 Gy TBI and fludarabine. In case of active disease, sequential conditioning regimens were used such as melphalan followed by 8 Gy TBI and fludarabine for AML or fludarabine with amsacrine and cytarabine (FLAMSA) followed by 12 Gy TBI for ALL, respectively. Prophylaxis for graft-versus-host disease and supportive care including anti-infective prophylaxis with antibiotics, anti-viral and anti-fungal drugs, growth factors and transfusion were provided according to standard of care procedures.
Follow-up and toxicity assessment
Follow-up procedures were scheduled according to national and international recommendations. To ensure an extensive posttransplant care, multidisciplinary long-term follow-up was initiated 12 months after allo-HSCT and successful engraftment. Follow-up procedures included graft-versus-host disease (GVHD) screening, serum chemistry, including thyroid function testing (thyroid-stimulating hormone levels [TSH], free triiodothyronine [fT3], and thyroxine [T4]), screening for hypogonadism (in men: baseline luteinizing hormone [LH] and testosterone levels; in women: baseline follicle stimulating hormone [FSH], LH and 17β-estradiol levels) and cortisol levels if necessary, assessment of liver function (bilirubin, aspartate aminotransferase [AST], alanine aminotransferase [ALT], alkaline phosphatase [ALP], hepatitis c virus [HCV] and hepatitis B virus [HBV] screening), iron metabolism (serum iron, ferritin, transferrin, haptoglobin, folic acid, vitamin B12, total iron binding capacity, transferrin saturation, soluble transferrin receptor), triglycerides, high-density lipoprotein (HDL) and low-density lipoprotein (LDL) levels. Blood count including lymphocyte subpopulations and reticulocytes. Evaluation of renal function with serum creatinine, blood urea nitrogen, and 24 h urine analysis including creatinine clearance, total protein, albumin and electrolytes.
Remission control is performed via bone marrow examination including chimerism monitoring and measurement of minimal residual disease (MRD). Computed tomography (CT) scans were performed as necessary.
Instrumental diagnostics include an electrocardiogram, echocardiogram, pulmonary function test, and diffusion capacity as well as baseline bone density testing.
Ophthalmologic examination includes assessment of visual acuity, fundoscopic examination and intraocular pressure measurement. Regular dermatological inspections are obligatory. Furthermore, dental health care, smoking cessation and vaccinations are recommended.
Follow-up examinations were initially performed every 3 months and later every 6 or 12 months, or according to individual risk. Toxicity was evaluated according to the Common Terminology Criteria for Adverse Events (CTCAE) version 5.0.
Statistical analyses
In this retrospective analysis, we used Spearman’s correlation coefficient for the assessment of the association between the variables. Time-to-event data were plotted according to the Kaplan–Meier method, while significance was calculated using the log-rank test. Overall survival was defined as the time from first fraction of radiotherapy to death from any cause or last follow-up. Event-free survival was defined as time from first radiotherapy to death from any cause, recurrence of the hematological disease or any other malignancy. The predefined primary endpoint of the study was event-free survival; secondary endpoints were overall survival, the cumulative incidence of secondary malignancies with death as competing risk and descriptive analyses and correlations of the above with patient and treatment characteristics. Impaired organ function was assessed using common parameters such as creatinine clearance, liver function parameters, or pulmonary function tests.
All tests were two sided and a p-value of 0.05 was considered statistically significant. The SPSS (version 25, IBM, Armonk, NY, USA) was used to perform all statistical analyses.
Results
Patient and treatment characteristics
We identified a total of 285 patients (female, n = 124, 43.5%) with hematopoietic diseases with a median age of 45 years (range: 16–65), who underwent a TBI as an integral part of the myelo- and immuno-ablative conditioning regimen followed by subsequent allo-HSCT at our institution between 1997 and 2015. The median follow-up for all patients was 49 months (range, 0–260 months) and 102 months (range, 36–251 months) for all patients alive. Patients were treated with allo-HSCT for acute myeloid leukemia (AML, n = 146), acute lymphoblastic leukemia (ALL, n = 91), lymphomas (n = 25) or other diseases as myelodysplastic syndrome or aplastic anemia (n = 23). The median radiotherapy dose applied was 10.5 Gy (range: 1.9–13.5 Gy, measured with in vivo dosimetry), with the most common dose of 8 Gy for AML and 12 Gy for ALL. The most commonly prescribed TBI dose was 12 Gy (n = 141, 49.5%) followed by 8 Gy (n = 90, 31.6%) and 4 Gy (n = 33, 11.6%). In all, 14 (4.9%) patients discontinued an ongoing series/TBI due to complications. In all, 238 patients (83.5%) received a total dose of at least 8 Gy. All characteristics are summarized in Table 1.
Clinical outcomes: disease control and survival
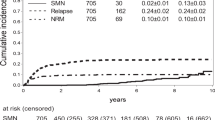
Overall survival (OS) at 1, 2, 5 and 10 years was 72.6, 64.6, 54.4 and 51.6%, respectively. The median OS for all patients was 120 months (Fig. 1). A total of 151 (52.9%) patients died during the observation period including 100 patients due to recurrence or progression of the underlying disease (66.2% of all deaths), 8 patients due to infections (5.3%), 5 patients due to acute GVHD (3.3%) and 38 patients following other transplant-related events (25.1%). Two patients died from unknown causes. This results in a cumulative incidence of treatment-related mortality of 17.9% for the complete cohort. Sex (p = 0.669) and age (p = 0.188) had no impact on survival in our cohort. Patients treated with TBI for the diagnoses of ALL (n = 91), AML (n = 146) or other hematological diseases (n = 48) had a comparable OS without significant differences (p = 0.559). In all, 89 patients (31.2%) had recurrent or refractory disease after allo-HSCT, of whom 46 patients relapsed in the first 24 months at a median time of 7 months (range 2–24). Furthermore, 56 events were reported in the first 6 months, 82 within 12 months, and 105 within 24 months. After 5 years, 142 events were recorded.
Patients who received a dose of ≥ 8 Gy had a superior overall (p = 0.025) and event-free survival (p = 0.030), respectively. There was no further significance between radiation-dose and overall survival for higher dose cut-offs. Altogether, significantly less events (any death, recurrent disease or secondary malignancies) were reported in the group of patients who received at least 8 Gy (p = 0.03; Fig. 2).
Secondary malignancies
Thirty-five secondary malignancies occurred in 33 patients after 7–224 months (Fig. 1). The cumulative incidence of secondary malignancies for all patients after 5 and 10 years was 5.6 and 8.8%, respectively. Of the 35 secondary malignancies, 28 were solid and 7 were hematologic. Two patients developed more than one secondary malignancy (nonmelanoma skin cancer and colorectal cancer or gynecological cancer, respectively). Skin cancer (n = 8) and hematological malignancies (n = 7) occurred most frequently followed by thyroid (n = 3), colon (n = 3) and gynecological cancers (n = 3). A detailed overview of the occurring secondary malignancies is given in Fig. 3. Seven patients died because of secondary malignancies after a median time of 10 months (range 1–64 months). In this cohort, there was no relationship between the risk of secondary malignancies and sex or age at different cut offs (median and 60 years) (p = 0.809, p = 0.932 and p = 0.546, respectively). After univariate analysis, the risk of developing a secondary malignancy and the OS among patients undergoing a TBI were both associated with the total dose of irradiation: lower doses (cut-off ≤ 8 Gy, < 10 Gy and < 12 Gy) were significantly correlated with an increased risk of secondary malignancies (p = 0.003, p = 0.004 and p = 0.040, respectively). Patients diagnosed with AML or other myeloid diseases had also an increased risk of secondary malignancies (p = 0.048) when compared to patients with ALL or lymphoma (Fig. 4).
After multivariate analysis, no significance remained. A summary of the significant values according to the univariate and the corresponding multivariate analyses for the most important time-to-event endpoints can be found in Table 2.
Other toxicity
In all, 220 nonmalignant chronic morbidities were reported as depicted in Fig. 5. In total, 144 patients developed severe organ damage (50.5%). Most prevalent nonmalignant late toxicity was decline in renal function in 33% of the patients, followed by endocrine disorders in 14.4% and a decline in pulmonary function in 13.3%. No significant correlation was observed between the impairment of renal and pulmonary function and increasing TBI dose. Any acute toxicity including acute GVHD grade > II requiring systemic treatment, was reported in 92 cases (32.3%; supplementary Tables 1 and 2).
Discussion
The present study presents epidemiological data, oncological outcome and late toxicity of a large cohort treated with TBI in a tertiary cancer center. While overall survival exceeded 50% at 10 years, we report considerable rates of sequelae, including secondary malignancies. These late effects did not seem to be correlated with higher irradiation doses in an intermediate dose range (6–12 Gy), while higher TBI doses were significantly correlated with improved outcomes. Therefore, overall and event-free survival were significantly higher in patients receiving at least 8 Gy total dose (p = 0.025 and p = 0.030) without further improvements for any dose above.
Based on the limited number of studies, most of which are underpowered and involve small and heterogeneous cohorts, a conflicting and inconsistent literature emerges, leaving the optimal conditioning regimen elusive [32,33,34]. Combining two modalities is a common approach in oncology. Overall, there is a growing body of evidence of using reduced-intensity and chemotherapy-based conditioning regimens to increase overall survival and reduce treatment-related mortality. After reduced-intensity conditioning regimens, a higher relapse rate has been reported in general, while overall survival increased due to a significant decrease in treatment-related mortality [35]. The only prospective study (BMT CTN 0901) comparing myeloablative versus reduced-intensity, chemotherapy-based conditioning regimens has been closed due to a higher relapse rate and only a modest decrease in treatment-related mortality in the reduced-intensity arm [36]. Although only 8 of 272 AML or MDS patients underwent a myeloablative conditioning regimen including total body irradiation, the study supports the use of myeloablative conditioning regimens for patients who are able to tolerate this intensified treatment [36].
Another prospective, open label randomized phase III trial compared an 8 Gy TBI and fludarabine-based conditioning regimen with a 12 Gy TBI and cyclophosphamide-based conditioning regimen. Within the 8 Gy TBI + Cy cohort, there was a reduced acute toxicity with no change the in incidence of nonrelapse mortality and survival [37]. Long-term results revealed that the reduced-intensity conditioning regime with moderately reduced total body irradiation doses was associated with lower acute morbidity and toxicity, while the risk of late relapse remained unaffected. Notably, the study has failed the recruitment and was closed early [38].
Therefore, reduced-intensity conditioning regimens, especially for older patients, became predominate [39]. Malard et al. compared two cohorts receiving allo-HSCT between 1983 and 2010. Patients in the cohort between 2001 and 2010 were older and reduced-intensity conditioning became more prevalent. Overall survival was improved by a significantly decreased nonrelapse mortality, while relapse rate was increased [40]. These results strengthen the aforementioned assumption, while this could further be a result of improvements in donor selection like high-resolution HLA matching as well as supportive care and thereby reducing the risk of posttransplant complications [40, 41].
In another retrospective cohort study, Gooptu et al. evaluated the outcome of 387 patients undergoing a myeloablative conditioning regimen consisting of cyclophosphamide and TBI (CyTBI) versus fludarabine and busulfan (FluBu). Again, mediated by significantly lower treatment-related mortality and similar relapse rates, a significantly improved overall survival was demonstrated in the chemotherapy-only-based group [42]. In our institution, the application of cyclophosphamide was largely omitted in favor of fludarabine in combination with 8 Gy TBI.
About one third (n = 91, 31.9%) of the patients in the present cohort were diagnosed with ALL. Despite the assumptive paradigm shift towards chemotherapy-only conditioning, there may be a larger impact of TBI-based conditioning regimens on lymphoblastic neoplasms. Therefore, the advantage of TBI-based conditioning in ALL appears to be more relevant and is supported by more robust data [43,44,45,46]. In this regard, a recent multinational, randomized trial has shown the superiority of total body irradiation plus etoposide compared with chemotherapy-based conditioning in childhood ALL [47]. Grün et al. further reported on long-term outcome, toxicities, and secondary malignancies in a cohort of 109 children and adolescents treated with 12 Gy TBI predominantly for ALL. In this recent analysis, the authors confirmed TBI-based conditioning regimens as feasible and effective treatment modality in the management of childhood leukemia. A broad but manageable spectrum of long-term toxicities occurred in 43% of all patients, while secondary malignancies (n = 3, 2.8%) represent a so far rare event but require a close follow-up [48].
Particularly in childhood and in younger adults, TBI-based conditioning is an evident risk factor for long-term complications and secondary malignancies [9, 49, 50].
Nevertheless, the risk of secondary malignancies in adults is assumed to be considerably lower. In the present study, 35 (12.3%) secondary malignancies occurred in 33 individuals after a median time of 5.8 years (70 months). Seven patients died as a consequence of the secondary malignancy. These findings are comparable to earlier reports [8,9,10, 15]. Furthermore, our study showed that patients diagnosed with AML or other myeloid diseases had also an increased risk of secondary malignancies (p = 0.048) compared to patients with ALL or high-grade lymphomas. These findings are previously described by Bhatia and others, who similarly reported a higher incidence of secondary malignancies in patients with myeloid neoplasms [51].
Intriguingly, there was an inverse correlation between total dose and the incidence of secondary malignancies. In a recent, comprehensive analysis among 4905 allo-HSCT survivors performed at the Fred Hutchinson Cancer Research Center in Seattle, the investigators demonstrated a strong effect of TBI dose and dose fractionation on the risk of the development of secondary malignancies. Patients exposed to single fraction TBI (6–10 Gy) had the highest standardized incidence ratio of secondary malignancies [50]. Fractionation could lower this risk significantly, while excessive dose escalation (13.2–17.5 Gy) offsets this effect. Moreover, for intermediate doses the risk of secondary malignancies was slightly attenuated but still significantly increased compared with low dose TBI (2–4 Gy) or chemotherapy-based conditioning regimes [50]. Of note, no significant difference was reported after low-dose TBI compared with chemotherapy-only conditioning regimens. Ultimately, these results verify a strong dose- and fractionation-dependent effect of total body irradiation as a major risk factor for secondary malignancies [52]. In our study, the majority of patients received an intermediate dose with 8 or 12 Gy TBI. Only a minority were treated with low-dose TBI (4 Gy) and are therefore underrepresented. In a considerable number of patients, lower doses were administered due to complications, while higher doses were initially prescribed. Nevertheless, there was no extreme dose gradient. In addition, lower doses were most commonly prescribed for AML patients who are at increased baseline-risk for developing secondary malignancies compared to ALL patients (8 vs. 12 Gy). These could be two explanations for the inverse relationship between the applied dose and the occurrence of secondary malignancies, in contrast to the anticipated and suggestive positive relationship. These results stress the fact that the applied radiotherapy dose might only play a subordinate role in the occurrence of secondary malignancies.
In terms of further toxicity, we have reported numerous nonmalignant sequela. Pulmonary and renal toxicity was more prevalent in individuals receiving a lower dose, which might be partially explained by the use of individual lung shields for doses beyond 8 Gy highlighting its importance [53]. However, other explanations, such as an increased age or fludarabine-based chemotherapy regimens in patients with lower TBI dose, may contribute to pulmonary toxicity and further disguise the impact of radiation dose and lung shielding. In this context, Oertel et al. have presented two recent analyses focusing on long-term toxicities after TBI [54, 55]. In partial concordance with our results, they could not detect any difference in pulmonary toxicity after 8 or 12 Gy TBI, highlighting the complex interplay of various factors influencing toxicity [54]. In addition, the authors were able to provide a comprehensive analysis of long-term sequelae in a large cohort of patients who underwent TBI demonstrating a significantly higher rate of ocular toxicities (p = 0.013) and severe mucositis (p < 0.001) in patients treated with 12 Gy TBI. No higher rate of other side effects was observed in the 12 Gy group, consistent with our findings [55].
There are several limitations that are not exclusive to the retrospective analysis of this single-center experience. We are not able to report all secondary malignancies, while no clear distinction was made between posttransplant lymphoproliferative disorders (PTLD), hematologic and solid malignancies. Moreover, some of the documentation of long-term toxicities is certainly incomplete. With a median follow-up of only 102 months, there will be more events to report in future. Chronic GVHD is a major risk factor for skin cancer [56], which may be considered outside this general analysis. In addition, patients’ personal risk behavior, such as smoking and alcohol consumption, could not be recorded [57, 58]. Surviving allo-HSCT should result in an extensive posttransplant cancer screening anyway [59, 60]. Furthermore, numerous conditioning regimens and protocols were applied during our observation period leading to a heterogeneous cohort. The different chemotherapy regimens will have contributed significantly to survival and toxicities, although we did not include this in our analysis. The inclusion of discontinued TBI series may have further biased the results. We must emphasize that we cannot draw a final conclusion and it is not possible to prove any dose dependency, reflecting the complex interplay of numerous factors influencing outcome and toxicity. On the other hand, within the dose range used in our cohort, there appears to be a correlation between a higher TBI dose and disease-specific survival. However, it seems highly unlikely that a higher TBI dose has a beneficial effect on survival in the intermediate dose ranges, and these results are subject to confounding and substantial bias. No decisive and critical difference in long-term toxicity was observed when comparing 8 Gy and 12 Gy TBI concepts. In summary, lower treatment-related mortality has led to significantly improved overall survival, but posttransplant relapse remains the most common cause of treatment failure and death in the last year. Still, TBI continues to be an integral part of many treatment concepts and should be further pursued. Balancing treatment-related mortality and disease-specific survival remains a critical consideration in finding an optimal and individualized conditioning regimen, as long-term toxicities continue to impact quality of life.
References
Paix A, Antoni D, Waissi W, Ledoux M‑P, Bilger K, Fornecker L et al (2018) Total body irradiation in allogeneic bone marrow transplantation conditioning regimens: a review. Crit Rev Oncol Hematol 123:138–148
Thomas ED, Buckner CD, Banaji M et al (1977) One hundred patients with acute leukemia treated by chemotherapy, total body irradiation, and allogeneic marrow transplantation. Blood 49(4):511–533
Thomas ED, Buckner CD, Clift RA, Fefer A, Johnson FL, Neiman PE et al (1979) Marrow transplantation for acute nonlymphoblastic leukemia in first remission. N Engl J Med 301(11):597–599
Quast U (2006) Whole body radiotherapy: a TBI-guideline. J Med Phys 31(1):5–12
Blume KG, Beutler E, Bross KJ, Chillar RK, Ellington OB, Fahey JL et al (1980) Bone-marrow ablation and allogeneic marrow transplantation in acute leukemia. N Engl J Med 302(19):1041–1046
Shank B, Chu FC, Dinsmore R, Kapoor N, Kirkpatrick D, Teitelbaum H et al (1983) Hyperfractionated total body irradiation for bone marrow transplantation. Results in seventy leukemia patients with allogeneic transplants. Int J Radiat Oncol Biol Phys 9(11):1607–1611
Sresty NVNM, Gudipudi D, Krishnam Raju A, Anil Kumar T, Lakshmi VRP, Srikanth G, Narasimha M (2021) Total body irradiation of bone marrow transplant using helical tomotherapy with a focus on the quality of dose contribution at junction target volumes. Strahlenther Onkol 197(8):722–729. https://doi.org/10.1007/s00066-021-01769-2
Curtis RE, Rowlings PA, Deeg HJ, Shriner DA, Socíe G, Travis LB et al (1997) Solid cancers after bone marrow transplantation. N Engl J Med 336(13):897–904
Baker KS, DeFor TE, Burns LJ, Ramsay NKC, Neglia JP, Robison LL (2003) New malignancies after blood or marrow stem-cell transplantation in children and adults: incidence and risk factors. J Clin Oncol 21(7):1352–1358
Hasegawa W, Pond GR, Rifkind JT, Messner HA, Lau A, Daly AS et al (2005) Long-term follow-up of secondary malignancies in adults after allogeneic bone marrow transplantation. Bone Marrow Transplant 35(1):51–55
Bölling T, Kreuziger DC, Ernst I, Elsayed H, Willich N (2011) Retrospective, monocentric analysis of late effects after total body irradiation (TBI) in adults. Strahlenther Onkol 187(5):311–315
Quast U, Dutreix A, Broerse JJ (1990) Late effects of total body irradiation in correlation with physical parameters. Radiother Oncol 18(1):158–162
Lowsky R, Lipton J, Fyles G, Minden M, Meharchand J, Tejpar I et al (1994) Secondary malignancies after bone marrow transplantation in adults. J Clin Oncol 12(10):2187–2192
Rizzo JD, Curtis RE, Socié G, Sobocinski KA, Gilbert E, Landgren O et al (2009) Solid cancers after allogeneic hematopoietic cell transplantation. Blood 113(5):1175–1183
Forrest DL, Nevill TJ, Naiman SC, Le A, Brockington DA, Barnett MJ et al (2003) Second malignancy following high-dose therapy and autologous stem cell transplantation: incidence and risk factor analysis. Bone Marrow Transplant 32(9):915–923
Danylesko I, Shimoni A (2018) Second malignancies after hematopoietic stem cell transplantation. Curr Treat Options Oncol 19(2):9
Scott BL, Sandmaier BM, Storer B, Maris MB, Sorror ML, Maloney DG et al (2006) Myeloablative vs nonmyeloablative allogeneic transplantation for patients with myelodysplastic syndrome or acute myelogenous leukemia with multilineage dysplasia: a retrospective analysis. Leukemia 20(1):128–135
Ringdén O, Labopin M, Ehninger G, Niederwieser D, Olsson R, Basara N et al (2009) Reduced intensity conditioning compared with myeloablative conditioning using unrelated donor transplants in patients with acute myeloid leukemia. J Clin Oncol 27(27):4570–4577
Gyurkocza B, Storb R, Storer BE, Chauncey TR, Lange T, Shizuru JA et al (2010) Nonmyeloablative allogeneic hematopoietic cell transplantation in patients with acute myeloid leukemia. J Clin Oncol 28(17):2859–2867
Gooley TA, Chien JW, Pergam SA, Hingorani S, Sorror ML, Boeckh M et al (2010) Reduced mortality after allogeneic hematopoietic-cell transplantation. N Engl J Med 363(22):2091–2101
Hahn T, McCarthy PL, Hassebroek A, Bredeson C, Gajewski JL, Hale GA et al (2013) Significant improvement in survival after allogeneic hematopoietic cell transplantation during a period of significantly increased use, older recipient age, and use of unrelated donors. J Clin Oncol 31(19):2437–2449
Mohty M, Malard F, Savani BN (2015) High-dose total body irradiation and myeloablative conditioning before allogeneic hematopoietic cell transplantation: time to rethink? Biol Blood Marrow Transplant 21(4):620–624
Hamilton BK (2018) TBI: to be (irradiated) or not to be? That remains the question. Biol Blood Marrow Transplant 24(8):1535–1536
Shi-Xia X, Xian-Hua T, Hai-Qin X, Bo F, Xiang-Feng T (2010) Total body irradiation plus cyclophosphamide versus busulphan with cyclophosphamide as conditioning regimen for patients with leukemia undergoing allogeneic stem cell transplantation: a meta-analysis. Leuk Lymphoma 51(1):50–60
Shimoni A, Shem-Tov N, Chetrit A, Volchek Y, Tallis E, Avigdor A et al (2013) Secondary malignancies after allogeneic stem-cell transplantation in the era of reduced-intensity conditioning; the incidence is not reduced. Leukemia 27(4):829–835
Gyurkocza B, Sandmaier BM (2014) Conditioning regimens for hematopoietic cell transplantation: one size does not fit all. Blood 124(3):344–353
Ramm U, Licher J, Moog J, Scherf C, Kara E, Böttcher H‑D et al (2008) In vivo dosimetry with semiconducting diodes for dose verification in total-body irradiation. A 10-year experience. Strahlenther Onkol 184(7):376–380
Döhner H, Estey EH, Amadori S, Appelbaum FR, Büchner T, Burnett AK, Dombret H, Fenaux P, Grimwade D, Larson RA, Lo-Coco F, Naoe T, Niederwieser D, Ossenkoppele GJ, Sanz MA, Sierra J, Tallman MS, Löwenberg B, Bloomfield CD (2010) Diagnosis and management of acute myeloid leukemia in adults: recommendations from an international expert panel, on behalf of the European LeukemiaNet. European LeukemiaNet. Blood 115(3):453–474. https://doi.org/10.1182/blood-2009-07-235358
Koreth J, Schlenk R, Kopecky KJ, Honda S, Sierra J, Djulbegovic BJ et al (2009) Allogeneic stem cell transplantation for acute myeloid leukemia in first complete remission: systematic review and meta-analysis of prospective clinical trials. JAMA 301(22):2349–2361
Loke J, Malladi R, Moss P, Craddock C (2020) The role of allogeneic stem cell transplantation in the management of acute myeloid leukaemia: a triumph of hope and experience. Br J Haematol 188(1):129–146
Gökbuget N, Stanze D, Beck J, Diedrich H, Horst HA, Hüttmann A, Kobbe G, Kreuzer KA, Leimer L, Reichle A, Schaich M, Schwartz S, Serve H, Starck M, Stelljes M, Stuhlmann R, Viardot A, Wendelin K, Freund M, Hoelzer D, German Multicenter Study Group for Adult Acute Lymphoblastic Leukemia (2012) Outcome of relapsed adult lymphoblastic leukemia depends on response to salvage chemotherapy, prognostic factors, and performance of stem cell transplantation. Blood 120(10):2032–2041. https://doi.org/10.1182/blood-2011-12-399287
Shimoni A, Hardan I, Shem-Tov N, Yeshurun M, Yerushalmi R, Avigdor A et al (2006) Allogeneic hematopoietic stem-cell transplantation in AML and MDS using myeloablative versus reduced-intensity conditioning: the role of dose intensity. Leukemia 20(2):322–328
Flynn CM, Hirsch B, Defor T, Barker JN, Miller JS, Wagner JE et al (2007) Reduced intensity compared with high dose conditioning for allotransplantation in acute myeloid leukemia and myelodysplastic syndrome: a comparative clinical analysis. Am J Hematol 82(10):867–872
Lioure B, Béné MC, Pigneux A, Huynh A, Chevallier P, Fegueux N et al (2012) Early matched sibling hematopoietic cell transplantation for adult AML in first remission using an age-adapted strategy: long-term results of a prospective GOELAMS study. Blood 119(12):2943–2948
Cooper JP, Storer BE, Granot N, Gyurkocza B, Sorror ML, Chauncey TR et al (2021) Allogeneic hematopoietic cell transplantation with non-myeloablative conditioning for patients with hematologic malignancies: Improved outcomes over two decades. Haematologica 106(6):1599–1607. https://doi.org/10.3324/haematol.2020.248187
Scott BL, Pasquini MC, Logan BR, Wu J, Devine SM, Porter DL et al (2017) Myeloablative versus reduced-intensity hematopoietic cell transplantation for acute myeloid leukemia and myelodysplastic syndromes. J Clin Oncol 35(11):1154–1161
Bornhauser M, Kienast J, Trenschel R, Burchert A, Hegenbart U, Stadler M, Baurmann H, Schafer-Eckart K, Holler E, Kroger N, Schmid C, Einsele H, Kiehl MG, Hiddemann W, Schwerdtfeger R, Buchholz S, Dreger P, Neubauer A, Berdel WE, Ehninger G, Beelen DW, Schetelig J, Stelljes M (2012) Reduced-intensity conditioning versus standard conditioning before allogeneic haemopoietic cell transplantation in patients with acute myeloid leukaemia in first complete remission: a prospective, open-label randomised phase 3 trial. Lancet Oncol 13(10):1035–1044
Fasslrinner F, Schetelig J, Burchert A, Kramer M, Trenschel R, Hegenbart U, Stadler M, Schafer-Eckart K, Batzel M, Eich H, Stuschke M, Engenhart-Cabillic R, Krause M, Dreger P, Neubauer A, Ehninger G, Beelen D, Berdel WE, Siepmann T, Stelljes M, Bornhauser M (2018) Long-term efficacy of reduced-intensity versus myeloablative conditioning before allogeneic haemopoietic cell transplantation in patients with acute myeloid leukaemia in first complete remission: retrospective follow-up of an open-label, randomised phase 3 trial. Lancet Haematol 5(4):e161–e9
Pasquini MC, Zhu X Current uses and outcomes of hematopoietic stem cell transplantation: 2014 CIBMTR summary slides. https://www.cibmtr.org/referencecenter/slidesreports/summaryslides/Pages/index.apsx. Accessed 7 November 2021
Malard F, Chevallier P, Guillaume T, Delaunay J, Rialland F, Harousseau J‑L et al (2014) Continuous reduced nonrelapse mortality after allogeneic hematopoietic stem cell transplantation: a single-institution’s three decade experience. Biol Blood Marrow Transplant 20(8):1217–1223
Flomenberg N, Baxter-Lowe LA, Confer D, Fernandez-Vina M, Filipovich A, Horowitz M et al (2004) Impact of HLA class I and class II high-resolution matching on outcomes of unrelated donor bone marrow transplantation: HLA‑C mismatching is associated with a strong adverse effect on transplantation outcome. Blood 104(7):1923–1930
Gooptu M, Kim HT, Ho VT, Alyea EP, Koreth J, Armand P et al (2018) A comparison of the myeloablative conditioning regimen fludarabine/busulfan with cyclophosphamide/total body irradiation, for allogeneic stem cell transplantation in the modern era: a cohort analysis. Biol Blood Marrow Transplant 24(8):1733–1740
Balduzzi A, Dalle J‑H, Wachowiak J, Yaniv I, Yesilipek A, Sedlacek P et al (2019) Transplantation in children and adolescents with acute lymphoblastic leukemia from a matched donor versus an HLA-identical sibling: is the outcome comparable? Results from the international BFM ALL SCT 2007 study. Biol Blood Marrow Transplant 25(11):2197–2210
Peters C, Schrappe M, von Stackelberg A, Schrauder A, Bader P, Ebell W et al (2015) Stem-cell transplantation in children with acute lymphoblastic leukemia: a prospective international multicenter trial comparing sibling donors with matched unrelated donors-the ALL-SCT-BFM-2003 trial. J Clin Oncol 33(11):1265–1274
Dalle J‑H, Balduzzi A, Bader P, Lankester A, Yaniv I, Wachowiak J et al (2018) Allogeneic stem cell transplantation from HLA-mismatched donors for pediatric patients with acute lymphoblastic leukemia treated according to the 2003 BFM and 2007 international BFM studies: impact of disease risk on outcomes. Biol Blood Marrow Transplant 24(9):1848–1855
Marnitz S, Zich A, Martus P, Budach V, Jahn U, Neumann O et al (2014) Long-term results of total body irradiation in adults with acute lymphoblastic leukemia. Strahlenther Onkol 190(5):453–458
Peters C, Dalle J‑H, Locatelli F, Poetschger U, Sedlacek P, Buechner J et al (2021) Total body irradiation or chemotherapy conditioning in childhood ALL: a multinational, randomized, noninferiority phase III study. J Clin Oncol 39(4):295–307
Gruen A, Exner S, Kühl JS, von Stackelberg A, Budach V, Stromberger C, Boehmer D (2021) Total body irradiation as part of conditioning regimens in childhood leukemia—long-term outcome, toxicity, and secondary malignancies. Strahlenther Onkol. https://doi.org/10.1007/s00066-021-01810-4
Bhatia S, Sklar C (2002) Second cancers in survivors of childhood cancer. Nat Rev Cancer 2(2):124–132
Oeffinger KC, Bhatia S (2009) Second primary cancers in survivors of childhood cancer. Lancet 374(9700):1484–1485
Bhatia S, Louie AD, Bhatia R, O’Donnell MR, Fung H, Kashyap A et al (2001) Solid cancers after bone marrow transplantation. J Clin Oncol 19(2):464–471
Baker KS, Leisenring WM, Goodman PJ, Ermoian RP, Flowers ME, Schoch G et al (2019) Total body irradiation dose and risk of subsequent neoplasms following allogeneic hematopoietic cell transplantation. Blood 133(26):2790–2799
Stephens SJ, Thomas S, Rizzieri DA, Horwitz ME, Chao NJ, Engemann AM et al (2016) Myeloablative conditioning with total body irradiation for AML: balancing survival and pulmonary toxicity. Adv Radiat Oncol 1(4):272–280
Oertel M, Kittel C, Martel J, Mikesch J‑H, Glashoerster M, Stelljes M, Eich HT (2021) Pulmonary toxicity after total body irradiation—an underrated complication? Estimation of risk via normal tissue complication probability calculations and correlation with clinical data. Cancers (Basel) 13(12):2946
Oertel M, Martel J, Mikesch J‑H, Scobioala S, Reicherts C, Kröger K, Lenz G, Stelljes M, Eich HT (2021) The burden of survivorship on hematological patients-long-term analysis of toxicities after total body irradiation and allogeneic stem cell transplantation. Cancers (Basel) 13(22):5640
Leisenring W, Friedman DL, Flowers MED, Schwartz JL, Deeg HJ (2006) Nonmelanoma skin and mucosal cancers after hematopoietic cell transplantation. J Clin Oncol 24(7):1119–1126
Adhikari J, Sharma P, Bhatt VR (2015) Risk of secondary solid malignancies after allogeneic hematopoietic stem cell transplantation and preventive strategies. Future Oncol 11(23):3175–3185
Bhatia S, Francisco L, Carter A, Sun C‑L, Baker KS, Gurney JG et al (2007) Late mortality after allogeneic hematopoietic cell transplantation and functional status of long-term survivors: report from the bone marrow transplant survivor study. Blood 110(10):3784–3792
Chow EJ, Cushing-Haugen KL, Cheng G‑S, Boeckh M, Khera N, Lee SJ et al (2017) Morbidity and mortality differences between hematopoietic cell transplantation survivors and other cancer survivors. J Clin Oncol 35(3):306–313
Majhail NS, Rizzo JD, Lee SJ, Aljurf M, Atsuta Y, Bonfim C et al (2012) Recommended screening and preventive practices for long-term survivors after hematopoietic cell transplantation. Bone Marrow Transplant 47(3):337–341
Acknowledgements
All procedures performed in this study involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Funding
This project received no external funding.
Funding
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Contributions
All authors have approved publication of the present manuscript.
Corresponding author
Ethics declarations
Conflict of interest
K. Sieker, M. Fleischmann, M. Trommel, U. Ramm, J. Licher, G. Bug, H. Martin, H. Serve, C. Rödel and P. Balermpas declare that they have no competing interests.
Ethical standards
Ethical approval for this study was obtained from the institutional review board (approval number: 221/16).
Additional information
The authors Katharina Sieker and Maximilian Fleischmann contributed equally to the manuscript.
Availability of data and material
The datasets generated and/or analyzed during the current study are available from the corresponding author on reasonable request.
Supplementary Information
Supplemental Table 1:
Subgroup analysis of the occurrence of adverse events and sequelae according to the median dose applied to all patients (10.5 Gy). Patients who received low-dose total body irradiation (TBI) or have not completed TBI were excluded, assuming their poor performance status. Significant values are given in bold
Supplemental Table 2
Occurring disorders in health after treatment depending on the total dose for all patients
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Sieker, K., Fleischmann, M., Trommel, M. et al. Twenty years of experience of a tertiary cancer center in total body irradiation with focus on oncological outcome and secondary malignancies. Strahlenther Onkol 198, 547–557 (2022). https://doi.org/10.1007/s00066-022-01914-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00066-022-01914-5