Abstract
Purpose
For a large or symptomatic brain metastasis, resection and adjuvant radiotherapy are recommended. Hypofractionated stereotactic radiotherapy (HFSRT) is increasingly applied in patients with a limited number of lesions. Exact target volume definition is critical given the small safety margins. Whilst technical advances have minimized inaccuracy due to patient positioning and radiation targeting, little is known about changes in target volume. This study sought to evaluate potential changes in the resection cavity of a brain metastasis.
Methods
In all, 57 patients treated with HFSRT after surgical resection of one brain metastasis between 2008 and 2015 in our institution were included in this study. Gross tumor volume (GTV) of the initial metastasis and the volume of the resection cavity in the post-operative, planning, and follow-up MRIs were measured and compared.
Results
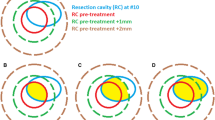
The mean cavity size decreased after surgery with the greatest change of −23.4% (±41.5%) occurring between post-operative MRI and planning MRI (p < 0.01). During this time period, the cavity volume decreased, remained stable, and increased in 79.1, 3.5, and 17.4%, respectively. A further decrease of −20.7% (±58.1%) was perceived between planning MRI and first follow-up (p < 0.01). No significant difference in pattern of change could be observed depending on the volume of initial GTV, size of the post-operative resection cavity, initial or post-resection FLAIR (fluid-attenuated inversion recovery) hyper-intensity, postsurgical ischemia, or primary tumor. The resection cavities of patients with post-operative ischemia were significantly larger than resection cavities of patients without ischemia.
Conclusion
The resection cavity seems to be very dynamic after surgery. Hence, it remains necessary to use very recent scans for treatment planning.
Zusammenfassung
Hintergrund
Die empfohlene Therapie für große und symptomatische Hirnmetastasen ist die chirurgische Resektion mit nachfolgender Bestrahlung der Resektionshöhle. Bei Patienten mit einer begrenzten Metastasenanzahl werden zunehmend stereotaktisch fraktionierte Konzepte (HFSRT) angewandt. Aufgrund der geringen Sicherheitssäume, die bei der HFSRT verwendet werden, ist die genaue Definition des Zielvolumens entscheidend. Während lagerungsbedingte Ungenauigkeiten durch technische Fortschritte weitgehend minimiert werden konnten, sind bisher wenige Informationen über Veränderungen der Zielvolumina bekannt. Ziel dieser Studie war es, Veränderungen der Resektionshöhlen von Hirnmetastasen zu untersuchen.
Methoden
In die Studie wurden 57 Patienten eingeschlossen, die zwischen 2008 und 2015 an unserer Klinik eine HFSRT der Resektionshöhle einer Hirnmetastase erhalten hatten. Das Tumorvolumen (GTV) der Metastase und die Volumina der Resektionshöhlen in der postoperativen Magnetresonanztomographie (MRT), im Planungs- und Nachsorge-MRT wurden gemessen und verglichen.
Ergebnisse
Die durchschnittliche Größe der Resektionshöhle nahm im Verlauf ab, wobei die größten Veränderungen von −23,4 % (±41,5 %) zwischen dem postoperativen MRT und dem Planungs-MRT auftraten (p < 0,01). In diesem Zeitraum wurde die Resektionshöhle in 79,1 % der Fälle kleiner, blieb in 3,5 % gleich und nahm in 17,4 % zu. Eine weitere signifikante Reduktion des Resektionshöhlenvolumens um −20,7 (±58,1 %) trat zwischen dem Planungs-MRT und der ersten Nachsorge auf. Signifikante Zusammenhänge zwischen der Veränderung der Resektionshöhlenvolumina und dem initialen Volumen der Metastase (GTV), der Größe der postoperativen Resektionskavität, der FLAIR(„fluid-attenuated inversion recovery‟)-Hyperintensität, der postoperativen Ischämie oder des Primärtumors konnten nicht nachgewiesen werden. Patienten mit postoperativer Ischämie hatten eine signifikant größere Resektionshöhle als Patienten ohne postoperative Ischämie.
Schlussfolgerung
Nach Operation von Hirnmetastasen treten Änderungen der Resektionshöhlenvolumina auf. Daher ist es notwendig, aktuelle Bildgebungen zur Bestrahlungsplanung zu verwenden.




Similar content being viewed by others
References
Al-Omair A, Soliman H, Xu W et al (2013) Hypofractionated stereotactic radiotherapy in five daily fractions for post-operative surgical cavities in brain metastases patients with and without prior whole brain radiation. Technol Cancer Res Treat 12(6):493–499
Atalar B, Choi CYH, Harsh GR et al (2013) Cavity volume dynamics after resection of brain metastases and timing of postresection cavity stereotactic radiosurgery. Neurosurgery 72(2):180–185 (discussion 185)
Blonigen BJ, Steinmetz RD, Levin L et al (2010) Irradiated volume as a predictor of brain radionecrosis after linear accelerator stereotactic radiosurgery. Int J Radiat Oncol Biol Phys 77(4):996–1001
Broemme J, Abu-Isa J, Kottke R et al (2013) Adjuvant therapy after resection of brain metastases. Frameless image-guided LINAC-based radiosurgery and stereotactic hypofractionated radiotherapy. Strahlenther Onkol 189(9):765–770
Brown PD, Ballman KV, Cerhan JH et al (2017) Postoperative stereotactic radiosurgery compared with whole brain radiotherapy for resected metastatic brain disease (NCCTG N107C/CEC·3). Lancet Oncol 18(8):1049–1060
Pérez-Larraya JG, Hildebrand J (2014) Chapter 77 – Brain metastases. In: Biller J, Ferro JM (Hrsg) Neurologic Aspects of Systemic Disease. Part III. Elsevier, Amsterdam, S 1143–1157
Doré M, Martin S, Delpon G et al (2017) Stereotactic radiotherapy following surgery for brain metastasis. Cancer Radiother 21(1):4–9
Frisk G, Svensson T, Bäcklund LM et al (2012) Incidence and time trends of brain metastases admissions among breast cancer patients in Sweden. Br J Cancer 106(11):1850–1853
Jarvis LA, Simmons NE, Bellerive M et al (2012) Tumor bed dynamics after surgical resection of brain metastases: Implications for postoperative radiosurgery. Int J Radiat Oncol Biol Phys 84(4):943–948
Johnson JD, Young B (1996) Demographics of brain metastasis. Neurosurg Clin N Am 7(3):337–344
Keller A, Doré M, Cebula H et al (2017) Hypofractionated Stereotactic radiation therapy to the resection bed for Intracranial metastases. Int J Radiat Oncol Biol Phys 99(5):1179–1189. https://doi.org/10.1016/j.ijrobp.2017.08.014
Kocher M, Soffietti R, Abacioglu U et al (2011) Adjuvant whole-brain radiotherapy versus observation after radiosurgery or surgical resection of one to three cerebral metastases: Results of the EORTC 22952-26001 study. J Clin Oncol 29(2):134–141
Kohutek ZA, Yamada Y, Chan TA et al (2015) Long-term risk of radionecrosis and imaging changes after stereotactic radiosurgery for brain metastases. J Neurooncol 125(1):149–156
Lamba N, Muskens IS, DiRisio AC et al (2017) Stereotactic radiosurgery versus whole-brain radiotherapy after intracranial metastasis resection: A systematic review and meta-analysis. Radiat Oncol 12(1):106
Lima LCS, Sharim J, Levin-Epstein R et al (2017) Hypofractionated Stereotactic Radiosurgery and radiotherapy to large resection cavity of metastatic brain tumors. World Neurosurg 97:571–579
Mahajan A, Ahmed S, McAleer MF et al (2017) Post-operative stereotactic radiosurgery versus observation for completely resected brain metastases. Lancet Oncol 18(8):1040–1048
Mehta MP, Tsao MN, Whelan TJ et al (2005) The American Society for Therapeutic Radiology and Oncology (ASTRO) evidence-based review of the role of radiosurgery for brain metastases. Int J Radiat Oncol Biol Phys 63(1):37–46
Minniti G, Clarke E, Lanzetta G et al (2011) Stereotactic radiosurgery for brain metastases. Radiat Oncol 6:48
NCCN (2017) NCCN clinical practice guidelines in oncology (NCCN guidelines ® ) central nervous system cancers. https://education.nccn.org/node/81831
Nussbaum ES, Djalilian HR, Cho KH et al (1996) Brain metastases. Histology, multiplicity, surgery, and survival. Cancer 78(8):1781–1788
O’Neill BP, Iturria NJ, Link MJ et al (2003) A comparison of surgical resection and stereotactic radiosurgery in the treatment of solitary brain metastases. Int J Radiat Oncol Biol Phys 55(5):1169–1176
Patchell RA, Tibbs PA, Walsh JW et al (1990) A randomized trial of surgery in the treatment of single metastases to the brain. N Engl J Med 322(8):494–500
Qin H, Wang C, Jiang Y et al (2015) Patients with single brain metastasis from non-small cell lung cancer equally benefit from stereotactic radiosurgery and surgery. Med Sci Monit 21:144–152
Schouten LJ, Rutten J, Huveneers HAM et al (2002) Incidence of brain metastases in a cohort of patients with carcinoma of the breast, colon, kidney, and lung and melanoma. Cancer 94(10):2698–2705
Shah JK, Potts MB, Sneed PK et al (2016) Surgical cavity constriction and local progression between resection and Adjuvant Radiosurgery for brain metastases. Cureus 8(4):e575
Specht HM, Kessel KA, Oechsner M et al (2016) HFSRT der Resektionshöhle bei Patienten mit Hirnmetastasen. Strahlenther Onkol 192(6):368–376
Spencer K, Hall A, Jain P (2014) Brain metastases. Clin Med (lond) 14(5):535–537
Steinmann D, Maertens B, Janssen S et al (2012) Hypofractionated stereotactic radiotherapy (hfSRT) after tumour resection of a single brain metastasis: Report of a single-centre individualized treatment approach. J Cancer Res Clin Oncol 138(9):1523–1529
van Leeuwen CM, Oei AL, Crezee J et al (2018) The alfa and beta of tumours. Radiat Oncol 13(1):96
Walker AE, Robins M, Weinfeld FD (1985) Epidemiology of brain tumors: The national survey of intracranial neoplasms. Baillieres Clin Neurol 35(2):219–226
Wang C‑C, Floyd SR, Chang C‑H et al (2012) Cyberknife hypofractionated stereotactic radiosurgery (HSRS) of resection cavity after excision of large cerebral metastasis: Efficacy and safety of an 800 cGy × 3 daily fractions regimen. J Neurooncol 106(3):601–610
Yamamoto M, Serizawa T, Shuto T et al (2014) Stereotactic radiosurgery for patients with multiple brain metastases (JLGK0901). Lancet Oncol 15(4):387–395
Acknowledgements
The authors thank our team of technicians for excellent patient care.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
S. Scharl, A. Kirstein, K.A. Kessel, M.-N. Duma, M. Oechsner, C. Straube, and S.E. Combs declare that they have no competing interests.
Ethical standards
All patients were treated in accordance with the Declaration of Helsinki. A written informed consent for the use of scientific data was obtained from all patients. This study was approved by the Ethics Committee of the Technical University Munich, Faculty of Medicine.
Rights and permissions
About this article
Cite this article
Scharl, S., Kirstein, A., Kessel, K.A. et al. Cavity volume changes after surgery of a brain metastasis—consequences for stereotactic radiation therapy. Strahlenther Onkol 195, 207–217 (2019). https://doi.org/10.1007/s00066-018-1387-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00066-018-1387-y
Keywords
- Resection cavity dynamics
- Hypofractionated stereotactic irradiation
- Neuro-oncology
- Adjuvant radiotherapy
- Constriction of the surgical bed