Abstract
Background
Radiotherapy (RT) is an established treatment for patients with primary and recurrent prostate cancer. Herein, the effects of definitive and salvage RT on the composition of lymphocyte subpopulations were investigated in patients with prostate cancer to study potential immune effects.
Patients and methods
A total of 33 prostate cancer patients were treated with definitive (n = 10) or salvage RT (n = 23) after biochemical relapse. The absolute number of lymphocytes and the distribution of lymphocyte subpopulations were analyzed by multiparameter flow cytometry before RT, at the end of RT, and in the follow-up period.
Results
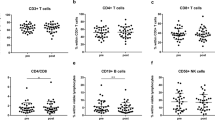
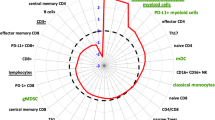
Absolute lymphocyte counts decreased significantly after RT in both patient groups and a significant drop was observed in the percentage of B cells directly after RT from 10.1 ± 1.3 to 6.0 ± 0.7% in patients with definitive RT and from 9.2 ± 0.8 to 5.8 ± 0.7% in patients with salvage RT. In contrast, the percentages of T and natural killer (NK) cells remained unaltered directly after RT in both patient groups. However, 1 year after RT, the percentage of CD3+ T cells was significantly lower in patients with definitive and salvage RT. The percentage of regulatory T cells was slightly upregulated in primary prostate cancer patients after definitive RT, but not after salvage RT.
Conclusion
Definitive and salvage RT exert similar effects on the composition of lymphocyte subpopulations in prostate cancer patients. Total lymphocyte counts are lower in both patient groups compared to healthy controls and further decreased after RT. B cells are more sensitive to definitive and salvage RT than T and NK cells.
Zusammenfassung
Hintergrund
Die Strahlentherapie (RT) ist eine bewährte Behandlung bim primären und rezidivierten Prostatakarzinoms. In dieser Studie wurde der Einfluss einer definitiven und Salvage RT auf die Zusammensetzung der Lymphozytensubpopulationen verglichen, um potenzielle Immuneffekte einer RT zu analysieren.
Patienten und Methoden
In die Studie wurden 33 Prostatakarzinompatienten eingeschlossen. Mit definitiver RT wurden 10 Patienten mit primärem Prostatakarzinom behandelt; 23 Patienten erhielten eine Salvage-RT nach biochemischem Rezidiv. Die absoluten Lymphozytenzahlen sowie die der Subpopulationen wurden vor RT, am Ende der RT und während des Follow-ups mittels Durchflusszytometrie analysiert.
Ergebnisse
Die absoluten Lymphozytenzahlen gingen in beiden Gruppen signifikant zurück. Direkt nach RT zeigte sich ein signifikanter Abfall der B‑Lymphozyten von 10,1 ± 1,3 % auf 6,0 ± 0,7 % bei Patienten mit definitiver RT und von 9,2 ± 0,8 % auf 5,8 ± 0,7 % bei Patienten mit Salvage-RT. Im Gegensatz dazu blieb der Anteil von T‑ und natürlichen Killerzellen (NK-Zellen) direkt nach RT in beiden Gruppen unverändert. Ein Jahr nach definitiver und Salvage-RT zeigte sich jedoch ein signifikanter Abfall der CD3+-T-Zellen. Der Anteil regulatorischer T‑Zellen war bei Patienten mit primärem Prostatakarzinom nach definitiver RT leicht erhöht, nicht jedoch nach Salvage-RT.
Schlussfolgerung
Die Verteilung der Lymphozytensubpopulationen nach definitiver oder Salvage-RT unterscheidet sich nicht signifikant in den Patientengruppen. Beide Gruppen weisen im Vergleich zu gesunden Kontrollen erniedrigte Gesamtlymphozytenwerte auf, die nach RT noch weiter absinken. B‑Zellen verhalten sich gegenüber einer definitiven und Salvage-RT sensibler als T‑ und NK-Zellen.


Similar content being viewed by others
References
Siegel RL, Miller KD, Jemal A (2016) Cancer statistics, 2016. CA Cancer J Clin 66(1):7–30. doi:10.3322/caac.21332
Ferlay J, Steliarova-Foucher E, Lortet-Tieulent J, Rosso S, Coebergh JW, Comber H, Forman D, Bray F (2013) Cancer incidence and mortality patterns in Europe: estimates for 40 countries in 2012. Eur J Cancer 49(6):1374–1403. doi:10.1016/j.ejca.2012.12.027
Pinkawa M, Piroth MD, Holy R, Djukic V, Klotz J, Krenkel B, Eble MJ (2011) Combination of dose escalation with technological advances (intensity-modulated and image-guided radiotherapy) is not associated with increased morbidity for patients with prostate cancer. Strahlenther Onkol 187(8):479–484. doi:10.1007/s00066-011-2249-z
Catton C, Milosevic M (2003) Salvage radiotherapy following radical prostatectomy. World J Urol 21(4):243–252. doi:10.1007/s00345-003-0360-1
Trock BJ, Han M, Freedland SJ, Humphreys EB, DeWeese TL, Partin AW, Walsh PC (2008) Prostate cancer-specific survival following salvage radiotherapy vs observation in men with biochemical recurrence after radical prostatectomy. JAMA 299(23):2760–2769. doi:10.1001/jama.299.23.2760
Pinkawa M (2015) Recent advances in definitive radiotherapy for prostate cancer. Eur Med J Oncol 3(1):41–48
Budach W, Sachkerer I (2014) Dose escalation: an update on randomised clinical trials. In: Geinitz R III (ed) Radiotherapy in prostate cancer. Medical radiology. Springer, Berlin, pp 89–93
Hou Z, Li G, Bai S (2015) High dose versus conventional dose in external beam radiotherapy of prostate cancer: a meta-analysis of long-term follow-up. J Cancer Res Clin Oncol 141(6):1063–1071. doi:10.1007/s00432-014-1813-1
Freedland SJ, Sutter ME, Dorey F, Aronson WJ (2003) Defining the ideal cutpoint for determining PSA recurrence after radical prostatectomy. Prostate-specific antigen. Urology 61(2):365–369
Geinitz H, Riegel MG, Thamm R, Astner ST, Lewerenz C, Zimmermann F, Molls M, Nieder C (2012) Outcome after conformal salvage radiotherapy in patients with rising prostate-specific antigen levels after radical prostatectomy. Int J Radiat Oncol Biol Phys 82(5):1930–1937. doi:10.1016/j.ijrobp.2011.03.003
Jereczek-Fossa BA, Orecchia R (2007) Evidence-based radiation oncology: definitive, adjuvant and salvage radiotherapy for non-metastatic prostate cancer. Radiother Oncol 84(2):197–215. doi:10.1016/j.radonc.2007.04.013
King CR (2012) The timing of salvage radiotherapy after radical prostatectomy: a systematic review. Int J Radiat Oncol Biol Phys 84(1):104–111. doi:10.1016/j.ijrobp.2011.10.069
Izawa JI (2009) Salvage radiotherapy after radical prostatectomy. Can Urol Assoc J 3(3):245–250
Malone S, Croke J, Roustan-Delatour N, Belanger E, Avruch L, Malone C, Morash C, Kayser C, Underhill K, Li Y, Malone K, Nyiri B, Spaans J (2012) Postoperative radiotherapy for prostate cancer: a comparison of four consensus guidelines and dosimetric evaluation of 3D-CRT versus tomotherapy IMRT. Int J Radiat Oncol Biol Phys 84(3):725–732. doi:10.1016/j.ijrobp.2011.12.081
Schaue D, Xie MW, Ratikan JA, McBride WH (2012) Regulatory T cells in radiotherapeutic responses. Front Oncol 2:90. doi:10.3389/fonc.2012.00090
Demaria S, Golden EB, Formenti SC (2015) Role of local radiation therapy in cancer immunotherapy. JAMA Oncol 1(9):1325–1332. doi:10.1001/jamaoncol.2015.2756
Bos PD, Plitas G, Rudra D, Lee SY, Rudensky AY (2013) Transient regulatory T cell ablation deters oncogene-driven breast cancer and enhances radiotherapy. J Exp Med 210(11):2435–2466. doi:10.1084/jem.20130762
Spiotto M, Fu YX, Weichselbaum RR (2016) The intersection of radiotherapy and immunotherapy: mechanisms and clinical implications. Sci Immunol 1(3). doi:10.1126/sciimmunol.aag1266
Formenti SC, Demaria S (2009) Systemic effects of local radiotherapy. Lancet Oncol 10(7):718–726. doi:10.1016/s1470-2045(09)70082-8
Sage EK, Schmid TE, Sedelmayr M, Gehrmann M, Geinitz H, Duma MN, Combs SE, Multhoff G (2016) Comparative analysis of the effects of radiotherapy versus radiotherapy after adjuvant chemotherapy on the composition of lymphocyte subpopulations in breast cancer patients. Radiother Oncol 118(1):176–180. doi:10.1016/j.radonc.2015.11.016
Deloch L, Derer A, Hartmann J, Frey B, Fietkau R, Gaipl US (2016) Modern radiotherapy concepts and the impact of radiation on immune activation. Front Oncol 6:141. doi:10.3389/fonc.2016.00141
Multhoff G, Gaipl US, Niedermann G (2012) The role of radiotherapy in the induction of antitumor immune responses. Strahlenther Onkol 188(Suppl 3):312–315. doi:10.1007/s00066-012-0206-0
Frey B, Rubner Y, Wunderlich R, Weiss EM, Pockley AG, Fietkau R, Gaipl US (2012) Induction of abscopal anti-tumor immunity and immunogenic tumor cell death by ionizing irradiation – implications for cancer therapies. Curr Med Chem 19(12):1751–1764
Cihan YB, Arslan A, Ergul MA (2013) Subtypes of white blood cells in patients with prostate cancer or benign prostatic hyperplasia and healthy individuals. Asian Pac J Cancer Prev 14(8):4779–4783
Pinkawa M, Ribbing C, Djukic V, Klotz J, Holy R, Eble MJ (2015) Early hematologic changes during prostate cancer radiotherapy predictive for late urinary and bowel toxicity. Strahlenther Onkol 191(10):771–777. doi:10.1007/s00066-015-0841-3
Gershkevitsh E, Rosenberg I, Dearnaley DP, Trott KR (1999) Bone marrow doses and leukaemia risk in radiotherapy of prostate cancer. Radiother Oncol 53(3):189–197
Stöver I, Feyer P (2010) Praxismanual Strahlentherapie. Springer, Berlin, p 31
Chapel H, Haeney M, Misbah S, Snowden N (2014) Essentials of clinical immunology, 6th edn. Wiley-Blackwell, Chichester
Multhoff G, Meier T, Botzler C, Wiesnet M, Allenbacher A, Wilmanns W, Issels RD (1995) Differential effects of ifosfamide on the capacity of cytotoxic T lymphocytes and natural killer cells to lyse their target cells correlate with intracellular glutathione levels. Blood 85(8):2124–2131
Belka C, Ottinger H, Kreuzfelder E, Weinmann M, Lindemann M, Lepple-Wienhues A, Budach W, Grosse-Wilde H, Bamberg M (1999) Impact of localized radiotherapy on blood immune cells counts and function in humans. Radiother Oncol 50(2):199–204
Sotosek S, Sotosek Tokmadzic V, Mrakovcic-Sutic I, Tomas MI, Dominovic M, Tulic V, Sutic I, Maricic A, Sokolic J, Sustic A (2011) Comparative study of frequency of different lymphocytes subpopulation in peripheral blood of patients with prostate cancer and benign prostatic hyperplasia. Wien Klin Wochenschr 123(23–24):718–725. doi:10.1007/s00508-011-0096-7
Nardone V, Botta C, Caraglia M, Martino EC, Ambrosio MR, Carfagno T, Tini P, Semeraro L, Misso G, Grimaldi A, Boccellino M, Facchini G, Berretta M, Vischi G, Rocca BJ, Barone A, Tassone P, Tagliaferri P, Del Vecchio MT, Pirtoli L, Correale P (2016) Tumor infiltrating T lymphocytes expressing FoxP3, CCR7 or PD-1 predict the outcome of prostate cancer patients subjected to salvage radiotherapy after biochemical relapse. Cancer Biol Ther. doi:10.1080/15384047.2016.1235666
Ness N, Andersen S, Valkov A, Nordby Y, Donnem T, Al-Saad S, Busund LT, Bremnes RM, Richardsen E (2014) Infiltration of CD8+ lymphocytes is an independent prognostic factor of biochemical failure-free survival in prostate cancer. Prostate 74(14):1452–1461. doi:10.1002/pros.22862
Tao CJ, Chen YY, Jiang F, Feng XL, Jin QF, Jin T, Piao YF, Chen XZ (2016) A prognostic model combining CD4/CD8 ratio and N stage predicts the risk of distant metastasis for patients with nasopharyngeal carcinoma treated by intensity modulated radiotherapy. Oncotarget 7(29):46653–46661. doi:10.18632/oncotarget.9695
Ordonez R, Henriquez-Hernandez LA, Federico M, Valenciano A, Pinar B, Lloret M, Bordon E, Rodriguez-Gallego C, Lara PC (2014) Radio-induced apoptosis of peripheral blood CD8 T lymphocytes is a novel prognostic factor for survival in cervical carcinoma patients. Strahlenther Onkol 190(2):210–216. doi:10.1007/s00066-013-0488-x
McClatchey KD (2002) Clinical laboratory medicine, 2nd edn. Lippincott Wiliams & Wilkins, Philadelphia
Acknowledgements
The authors thank Bernhard Haller for support with statistical analysis of the data.
Funding
This work was supported by EU-CELLEUROPE (315963); BMBF (Strahlenkompetenz, 02NUK038A; Innovative Therapies, 01GU0823; NSCLC, 16GW0030), German Cancer Consortium Radiation Oncology Group (DKTK-ROG); the DFG Cluster of Excellence: Munich Centre for Advanced Photonics (MAP); DFG SFB 824/2; INST 95/980-1 FUGG; INST 411/37-1 FUGG irradiation devices. Eva Sage was supported by the Medical Faculty of TUM as a fellow of the doctoral program “Translationale Medizin.” This work was supported by the German Research Foundation (DFG) and the Technische Universität München within the Open Access Publishing funding program.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
E.K. Sage, T.E. Schmid, H. Geinitz, M. Gehrmann, M. Sedelmayr, M.N. Duma, S.E. Combs, and G. Multhoff declare that they have no competing interests.
Caption Electronic Supplementary Material
66_2017_1144_MOESM1_ESM.docx
Supplementary table 1: List of all antibody combinations and chromophores which were used in the study. Antibody stainings which did not show any significant changes in the patient samples during the course of analysis were not shown in the results part.
66_2017_1144_MOESM2_ESM.jpg
Supplementary figure 1: Schematic representation of the gating procedure of the flow cytometry data. R1 lymphocyte gate, R2 granolocytes, R3 monocytes.
Rights and permissions
About this article
Cite this article
Sage, E.K., Schmid, T.E., Geinitz, H. et al. Effects of definitive and salvage radiotherapy on the distribution of lymphocyte subpopulations in prostate cancer patients. Strahlenther Onkol 193, 648–655 (2017). https://doi.org/10.1007/s00066-017-1144-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00066-017-1144-7