Abstract
Background
Inspiratory hyperoxia reduces tumor hypoxia, which is responsible for limited radiosensitivity of tumors. However, very little is known about the heterogeneity of intratumoral oxygenation during this supportive treatment. The study analyzes whether local hypoxia is still present during normobaric and hyperbaric inspiratory hyperoxia and whether the addition of CO2 to the inspiratory gas affects the spatial pO2 distribution.
Material and methods
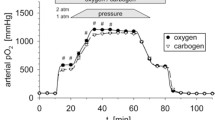
Tumor oxygenation of experimental DS-sarcomas in rats was assessed by polarographic needle electrodes at 1 and 2 atm (bar) environmental pressure during pure O2 or carbogen (95 % O2 + 5 % CO2) breathing. Up to 320 individual pO2 measurements were performed in a strictly oriented grid resulting in an oxygenation profile in a horizontal tumor layer.
Results
In the experimental tumors used the oxygenation showed pronounced heterogeneities with closely adjacent hypoxic and oxygenated regions. This heterogeneity was still visible under normobaric hyperoxia where large confluent hypoxic regions were detectable. At 1 atm, the addition of CO2 improved tumor oxygenation significantly (at least in large tumors). At 2 atm, only very small local regions of hypoxia were detected. However, under this condition hypercapnia had no impact on tumor oxygenation.
Conclusions
The data show that even under hyperbaric hyperoxia, hypoxic regions are detectable despite the average pO2 increased by a factor of 100. The results also clearly indicate that the oxygenation pattern improves disproportionally with increasing environmental pressure.
Zusammenfassung
Hintergrund
Inspiratorische Hyperoxie reduziert die Hypoxie im Tumorgewebe, die u. a. für eine verminderte Strahlensensibilität verantwortlich ist. Über die räumliche Heterogenität der intratumoralen Oxygenierung während inspiratorischer Hyperoxie ist jedoch wenig bekannt. Ziel der Untersuchung war daher die Analyse, inwieweit Hypoxie bei inspiratorischer Hyperoxie unter normo- oder hyperbaren Bedingungen weiterhin nachweisbar ist und ob darüber hinaus der Zusatz von CO2 zum Atemgas die räumliche pO2-Verteilung beeinflusst.
Material und Methoden
Die Tumoroxygenierung experimenteller DS-Sarkome der Ratte wurde mittels polarographischen Nadelelektroden bei einem Umgebungsdruck von 1 bzw. 2 atm (bar) gemessen. Die Versuchstiere atmeten hierbei Raumluft, reinen Sauerstoff oder Karbogen (95 % O2 + 5 % CO2). Bis zu 320 einzelne pO2-Werte wurden in jedem Tumor in einem gitterförmigen Raster ermittelt und pO2-Profile im Gewebe berechnet.
Ergebnisse
In dem untersuchten experimentellen Tumormodell fand sich eine ausgeprägte Heterogenität der Oxygenierung mit hypoxischen Regionen unmittelbar benachbart zu gut versorgten Geweberegionen. Diese Heterogenität mit großen zusammenhängenden hypoxischen Regionen ließ sich auch bei normobarer inspiratorischer Hyperoxie nachweisen. Bei normobarem Umgebungsdruck führte der Zusatz von CO2 zumindest in großen Tumoren zu einer signifikanten Verbesserung der Oxygenierung. Bei einem Druck von 2 atm ließen sich nur noch sehr kleine hypoxische Geweberegionen nachweisen. Hyperkapnie hatte unter diesen Bedingungen keinen Einfluss auf die Tumoroxygenierung.
Schlussfolgerungen
Die Ergebnisse belegen, dass auch bei hyperbarer Hyperoxie hypoxische Regionen im Tumor nachweisbar sind, obwohl der mittlere pO2 um den Faktor 100 anstieg. Die Daten zeigen deutlich, dass sich die Tumoroxygenierung mit zunehmendem Umgebungsdruck überproportional verbessert.







Similar content being viewed by others
References
Hoskin PJ, Rojas AM, Saunders MI et al (2009) Carbogen and nicotinamide in locally advanced bladder cancer: early results of a phase-III randomized trial. Radiother Oncol 91:120–125
Janssens GO, Bockel LW van, Doornaert PA et al (2014) Computed tomography-based tumour volume as a predictor of outcome in laryngeal cancer: results of the phase 3 ARCON trial. Eur J Cancer 50:1112–1119
Thews O, Kelleher DK, Vaupel P (2002) Dynamics of tumor oxygenation and red blood cell flux in response to inspiratory hyperoxia combined with different levels of inspiratory hypercapnia. Radiother Oncol 62:77–85
Janssens GO, Rademakers SE, Terhaard CH et al (2014) Improved recurrence-free survival with ARCON for anemic patients with laryngeal cancer. Clin Cancer Res 20:1345–1354
Rademakers SE, Hoogsteen IJ, Rijken PF et al (2015) Prognostic value of the proliferation marker Ki-67 in laryngeal carcinoma: results of the accelerated radiotherapy with carbogen breathing and Nicotinamide phase III randomized trial. Head Neck 37:171–176
Hoskin PJ, Rojas AM, Phillips H et al (2005) Acute and late morbidity in the treatment of advanced bladder carcinoma with accelerated radiotherapy, carbogen, and nicotinamide. Cancer 103:2287–2297
Becker A, Kuhnt T, Liedtke H et al (2002) Oxygenation measurements in head and neck cancers during hyperbaric oxygenation. Strahlenther Onkol 178:105–108
Brizel DM, Lin S, Johnson JL et al (1995) The mechanisms by which hyperbaric oxygen and carbogen improve tumour oxygenation. Br J Cancer 72:1120–1124
Shi Y, Lee CS, Wu J et al (2005) Effects of hyperbaric oxygen exposure on experimental head and neck tumor growth, oxygenation, and vasculature. Head Neck 27:362–369
Workman P, Aboagye EO, Balkwill F et al (2010) Guidelines for the welfare and use of animals in cancer research. Br J Cancer 102:1555–1577
Vaupel P, Schlenger K, Knoop C et al (1991) Oxygenation of human tumors: evaluation of tissue oxygen distribution in breast cancers by computerized O2 tension measurements. Cancer Res 51:3316–3322
Thews O, Vaupel P (1996) Relevant parameters for describing the oxygenation status of solid tumors. Strahlenther Onkol 172:239–243
Kelleher DK, Matthiensen U, Thews O et al (1996) Blood flow, oxygenation, and bioenergetic status of tumors after erythropoietin treatment in normal and anemic rats. Cancer Res 56:4728–4734
Vaupel P, Kallinowski F, Okunieff P (1989) Blood flow, oxygen and nutrient supply, and metabolic microenvironment of human tumors: a review. Cancer Res 49:6449–6465
Gray LH, Conger AD, Ebert M et al (1953) The concentration of oxygen dissolved in tissues at the time of irradiation as a factor in radiotherapy. Br J Radiol 26:638–648
Hall EJ, Giaccia AJ (2011) Radiobiology for the radiologist. Lippincott Williams & Wilkins, Philadelphia
Hou H, Dong R, Li H et al (2012) Dynamic changes in oxygenation of intracranial tumor and contralateral brain during tumor growth and carbogen breathing: a multisite EPR oximetry with implantable resonators. J Magn Reson 214:22–28
Padhani AR, Krohn KA, Lewis JS et al (2007) Imaging oxygenation of human tumours. Eur Radiol 17:861–872
Borges AR, Lopez-Larrubia P, Marques JB et al (2012) MR imaging features of high-grade gliomas in murine models: how they compare with human disease, reflect tumor biology, and play a role in preclinical trials. Am J Neuroradiol 33:24–36
Matsumoto K, Bernardo M, Subramanian S et al (2006) MR assessment of changes of tumor in response to hyperbaric oxygen treatment. Magn Reson Med 56:240–246
Hallac RR, Zhou H, Pidikiti R et al (2014) Correlations of noninvasive BOLD and TOLD MRI with pO2 and relevance to tumor radiation response. Magn Reson Med 71:1863–1873
Ding Y, Mason RP, McColl RW et al (2013) Simultaneous measurement of tissue oxygen level-dependent (TOLD) and blood oxygenation level-dependent (BOLD) effects in abdominal tissue oxygenation level studies. J Magn Reson Imaging 38:1230–1236
Bussink J, Kaanders JH, Rijken PF et al (1999) Vascular architecture and microenvironmental parameters in human squamous cell carcinoma xenografts: effects of carbogen and nicotinamide. Radiother Oncol 50:173–184
Bussink J, Kaanders JH, Rijken PF et al (2000) Changes in blood perfusion and hypoxia after irradiation of a human squamous cell carcinoma xenograft tumor line. Radiat Res 153:398–404
Henriques de FB, Zacharatou C, Galland-Girodet S et al (2015) Hypoxia imaging with [18F]-FMISO-PET for guided dose escalation with intensity-modulated radiotherapy in head-and-neck cancers. Strahlenther Onkol 191:217–224
Yaromina A, Thames H, Zhou X et al (2010) Radiobiological hypoxia, histological parameters of tumour microenvironment and local tumour control after fractionated irradiation. Radiother Oncol 96:116–122
Winther M, Alsner J, Tramm T et al (2013) Hypoxia-regulated gene expression and prognosis in loco-regional gastroesophageal cancer. Acta Oncol 52:1327–1335
Brennan DJ, Jirstrom K, Kronblad A et al (2006) CA IX is an independent prognostic marker in premenopausal breast cancer patients with one to three positive lymph nodes and a putative marker of radiation resistance. Clin Cancer Res 12:6421–6431
Müller A, Remmele S, Wenningmann I et al (2011) Analysing the response in R2* relaxation rate of intracranial tumours to hyperoxic and hypercapnic respiratory challenges: initial results. Eur Radiol 21:786–798
Acknowledgments
This study was supported by the Dr. med. h.c. Erwin Braun Foundation, Basel, Switzerland.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
O. Thews and P. Vaupel state that there are no conflicts of interest.
All national guidelines on the care and use of laboratory animals have been followed and the necessary approval was obtained from the relevant authorities.
Rights and permissions
About this article
Cite this article
Thews, O., Vaupel, P. Spatial oxygenation profiles in tumors during normo- and hyperbaric hyperoxia. Strahlenther Onkol 191, 875–882 (2015). https://doi.org/10.1007/s00066-015-0867-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00066-015-0867-6
Keywords
- Inspiratory hyperoxia
- Inspiratory hypercapnia
- Tumor oxygenation
- Spatial oxygen distribution
- Hyperbaric hyperoxia