Purpose:
Evaluation of late side effects and biochemical control (bNED) 5 years after three-dimensional radiotherapy with moderate, risk-adapted dose escalation.
Patients and Methods:
From 03/1999 to 07/2002, 486 patients have been registered in the prospective Austrian-German multicenter phase II trial (AUGE). 399 (82%) localized prostate cancer patients (T1–3 Nx/N0 M0) were evaluated. The low- and intermediate-risk groups were treated with 70 Gy, the high-risk group with 74 Gy, respectively. Additional hormonal therapy (HT) was recommended for intermediate- and high-risk group patients. Late toxicity (EORTC/RTOG) and bNED (ASTRO and Phoenix) were prospectively assessed.
Results:
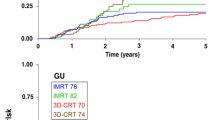
Median follow-up was 65 months. Distribution concerning risk groups (low-, intermediate-, high-risk group) showed 29%, 50% and 21% of patients, respectively. HT was given in 87% of patients. The 5-year actuarial rates of late side effects grade ≥ 2 for 70 Gy/74 Gy were 28%/30% (gastrointestinal; p = 0.73) and 19%/34% (urogenital; p = 0,06). The 5-year actuarial bNED rate stratified by risk groups (low-, intermediate-, high-risk group) was 74%, 66% and 50% (ASTRO), and 81%, 80% and 60% (Phoenix), respectively. Within multivariate analysis T-stage and initial prostate specific antigen were significant factors influencing bNED (ASTRO) whereas Gleason Score and duration of HT were not.
Conclusion:
Dose escalation within standard three-dimensional conformal radiotherapy (3D-CRT) up to a level of 74 Gy did not result in significantly increased gastrointestinal side effects, whereas urogenital side effects showed an increase close to significance. However, the total number of patients with severe toxicity was low. To achieve high tumor control rates with acceptable treatment-related morbidity, local doses of at least 74 Gy should be considered, in particular for intermediate- or high-risk patients applying 3D-CRT.
Ziel:
Bestimmung von Spätnebenwirkungen und biochemischer Kontrolle (bNED) nach risikoadaptierter Dosiseskalation im Rahmen einer prospektiven österreichisch-deutschen Phase-II-Multicenterstudie.
Patienten und Methodik:
Von 03/1999 bis 07/2002 wurden 486 Patienten mit Prostatakarzinom (T1–3 Nx/N0 M0) gemeldet, und 399 (82%) kamen zur Auswertung. Patienten der Niedrig- und Intermediärrisikogruppe wurden mit 70 Gy, die Hochrisikogruppe mit 74 Gy bestrahlt (Tabelle 1). Eine begleitende Hormontherapie wurde für Patienten der Intermediär- und Hochrisikogruppe empfohlen. Spätnebenwirkungen (EORTC/RTOG) und bNED (ASTRO/Phoenix) wurden ermittelt.
Ergebnisse:
Der mittlere Nachbeobachtungszeitraum betrug 65 Monate. Hinsichtlich der Risikogruppen (Niedrig-, Intermediär-, Hochrisikogruppe) fanden sich 29%, 50%, und 21% Patienten. Eine begleitende Hormontherapie erhielten 87% der Patienten. Detaillierte Patientendaten sind in Tabelle 2 aufgeführt. Die 5-Jahres-Raten an Spätnebenwirkungen Grad ≥ 2 für 70 Gy/74 Gy lagen bei 28%/30% (gastrointestinal; p = 0,73) und 19%/34% (urogenital; p = 0,06; Abbildungen 1 und 2). Die 5-Jahres-bNED-Raten entsprechend den Risikogruppen (Niedrig-, Intermediär-, Hochrisikogruppe) lagen bei 74%, 66% und 50% (ASTRO; Abbildung 3) bzw. 81%, 80% und 60% (Phoenix; Abbildung 4). In der multivariaten Analyse zeigten sich T-Stadium und initiales prostataspezifisches Antigen als signifikant bezüglich bNED (ASTRO) und Gleason-Score sowie die Dauer der Hormontherapie als nicht signifikant (Tabelle 4).
Schlussfolgerung:
Die Dosissteigerung auf 74 Gy führt zu keinen signifikant erhöhten Raten an gastrointestinalen Spätnebenwirkungen. Die Rate an urogenitalen Spätnebenwirkungen ist hingegen erhöht. Insgesamt finden sich jedoch nur wenige schwere Grad-3-Spätnebenwirkungen (Tabelle 3). Um respektable Tumorkontrollraten (Abbildung 5) zu erreichen, sollte, vor allem für Patienten der Intermediär- und Hochrisikogruppe, eine lokale Dosis von zumindest 74 Gy appliziert werden.
Similar content being viewed by others
References
American Society for Therapeutic Radiology and Oncology Consensus Panel. Consensus statement: guidelines for PSA following radiation therapy. Int J Radiat Oncol Biol Phys 1997;37:1035–41.
Cavey ML, Bayouth JE, Colman M, et al. IMRT to escalate the dose to the prostate while treating the pelvic nodes. Strahlenther Onkol 2005;181:431–41.
Cox JD, Stetz J, Pajak TF. Toxicity criteria of the Radiation Therapy Oncology Group (RTOG) and the European Organization for Research and Treatment of Cancer (EORTC). Int J Radiat Oncol Biol Phys 1995;31:1341–6.
Dearnaley DP, Sydes MR, Graham JD, et al. Escalated-dose versus standard-dose conformal radiotherapy in prostate cancer: first results from the MRC RT01 randomised controlled trial. Lancet Oncol 2007;8:475–87.
De Meerleer G, Vakaet L, De Gersem W, et al. Direct segment aperture and weight optimization for intensity-modulated radiotherapy of prostate cancer. Strahlenther Onkol 2004;180:136–43.
Dobler B, Mai S, Ross C, et al. Evaluation of possible prostate displacement induced by pressure applied during transabdominal ultrasound image acquisition. Strahlenther Onkol 2006;182:240–6.
Eade TN, Hanlon AL, Horwitz EM, et al. What dose of external-beam radiation is high enough for prostate cancer? Int J Radiat Oncol Biol Phys 2007;68:682–9.
Fiorino C, Fellin G, Rancati T, et al. Clinical and dosimetric predictors of late rectal syndrome after 3D-CRT for localized prostate cancer: preliminary results of a multicenter prospective study. Int J Radiat Oncol Biol Phys 2008;70:1130–7.
Gershkevitsh E, Clark CH, Staffurth J, et al. Dose to bone marrow using IMRT techniques in prostate cancer patients. Strahlenther Onkol 2005;181:172–8.
Gerstner N, Wachter S, Dorner D, et al. Significance of a rectal balloon as internal immobilization device in conformal radiotherapy of prostatic carcinoma. Strahlenther Onkol 1999;175:232–8.
Grosu AL, Piert M, Weber WA, et al. Positron emission tomography for radiation treatment planning. Strahlenther Onkol 2005;181:483–99.
Guckenberger M, Flentje M. Intensity-modulated radiotherapy (IMRT) of localized prostate cancer. A review and future perspectives. Strahlenther Onkol 2007;183:57–62.
Guckenberger M, Meyer J, Wilbert J, et al. Precision of image-guided radiotherapy (IGRT) in six degrees of freedom and limitations in clinical practice. Strahlenther Onkol 2007;183:307–13.
Hoskin PJ, Motohashi K, Bownes P, et al. High dose rate brachytherapy in combination with external beam radiotherapy in radical treatment of prostate cancer: initial results of a randomised phase three trial. Radiother Oncol 2007;84:114–20.
ICRU. Report 62: Prescribing, recording and reporting photon beam therapy (supplement to ICRU report 50). Bethesda: International Commission on Radiation Units and Measurements; 1999.
Jereczek-Fossa BA, Vavassori A, Fodor C, et al. Dose escalation for prostate cancer using the three-dimensional conformal dynamic arc technique: analysis of 542 consecutive patients. Int J Radiat Oncol Biol Phys 2008;71: 784–94.
Koelbl O, Schwab F, Bratengeier K, et al. Radiotherapy of prostate cancer with multileaf collimators (MLCs): optimization of the undulating dose distribution at the MLC edge. Strahlenther Onkol 2005;181:108–12.
Kuban DA, Tucker SL, Dong L, et al. Long-term results of the M.D. Anderson randomized dose-escalation trial for prostate cancer. Int J Radiat Oncol Biol Phys 2008;70:67–74.
Mock U, Bogner J, Georg D, et al. Comparative treatment planning on localized prostate carcinoma: conformal photon- versus proton-based radiotherapy. Strahlenther Onkol. 2005;181:448–55.
Peeters STH, Heemsbergen WD, Koper PCM, et al. Dose-response in radiotherapy for localized prostate cancer: results of the Dutch multicenter randomized phase III trial comparing 68 Gy of radiotherapy with 78 Gy. J Clin Oncol 2006;24:1990–6.
Pollack A, Hanlon AL, Horwitz EM, et al. Prostate cancer radiotherapy dose response: an update of the fox chase experience. J Urol 2004;171:1132–6.
Pollack A, Zagars G, Smith LG, et al. Preliminary results of a randomized radiotherapy dose-escalation study comparing 70 Gy with 78 Gy for prostate cancer. J Clin Oncol 2000;18:3904–11.
Pollack A, Zagars GK, Starkschall G, et al. Prostate cancer radiation dose response: results of the M.D. Anderson phase III randomized trial. Int J Radiat Oncol Biol Phys 2002;53:1097–105.
Reibenwein J. Risikoadaptierte konformale Strahlentherapie des Prostatakarzinoms mit neoadjuvanter Hormontherapie im Rahmen einer multi-center Phase II Studie: Korrelation von späten gastrointestinalen Nebenwirkungen und Rektoskopiebefunden mit Dosis-Volumen-Histogrammen des Rektums. Diplomarbeit 2005. Wien: Universitätsbibliothek, 2005: WJ-D2005-5.
Roach M3rd, Hanks G, Thames HJr, et al. Defining biochemical failure following radiotherapy with or without hormonal therapy in men with clinically localized prostate cancer: recommendations of the RTOG-ASTRO Phoenix Consensus Conference. Int J Radiat Oncol Biol Phys 2006;65:965–74.
Roach M3rd. Dose escalated external beam radiotherapy versus neoadjuvant androgen deprivation therapy and conventional dose external beam radiotherapy for clinically localized prostate cancer: do we need both? Strahlenther Onkol 2007183 (Spec No 2):26–8.
Sathaya JR, Davis IR, Julian JA, et al. Randomized trial comparing iridium implant plus external-beam radiation therapy with external-beam radiation therapy alone in node-negative locally advanced cancer of the prostate. J Clin Oncol 2005;23:1192–9.
Schulte RW, Slater JD, Rossi CJ, et al. Value and perspectives of proton radiation therapy for limited stage prostate cancer. Strahlenther Onkol 2000;176:3–8.
Shipley WU, Verhey LJ, Munzenrider JE, et al. Advanced prostate cancer: the results of a randomized comparative trial of high dose irradiation boosting with conformal protons compared with conventional dose irradiation using photons alone. Int J Radiat Oncol Biol Phys 1995;32:3–12.
Shu H-KG, Lee TT, Vigneault E, et al. Toxicity following high-dose three-dimensional conformal and intensity-modulated radiation therapy for clinically localized prostate cancer. Urology 2001;57:102–7.
Sterzing F, Schubert K, Sroka-Perez G, et al. Helical tomotherapy. Experiences of the first 150 patients in Heidelberg. Strahlenther Onkol 2008;184: 8–14.
Wachter S, Gerstner N, Goldner G, et al. Rectal sequelae after conformal radiotherapy of prostate cancer: dose-volume histograms as predictive factors. Radiother Oncol 2001;59:65–70.
Yeoh EE, Holloway R, Fraser RJ, et al. Hypofractionated versus conventionally fractionated radiation therapy for prostate carcinoma: updated results of a phase III randomized trial. Int J Radiat Oncol Biol Phys 2006;66: 1072–83.
Zelefsky MJ, Chan H, Hunt M, et al. Long-term outcome of high dose intensity modulated radiation therapy for patients with clinically localized prostate cancer. J Urol 2006;176:1415–9.
Zelefsky MJ, Leibel SA, Gaudin PB, et al. Dose escalation with three dimensional conformal radiation therapy affects the outcome in prostate cancer. Int J Radiat Oncol Biol Phys 1998;41:491–500.
Zelefsky MJ, Levin EJ, Hunt M, et al. Incidence of late rectal and urinary toxicities after three-dimensional conformal radiotherapy and intensity-modulated radiotherapy for localized prostate cancer. Int J Radiat Oncol Biol Phys 2008;70:1124–9.
Zelefsky MJ, Moughan J, Owen J, et al. Changing trends in national practice for external beam radiotherapy for clinically localized prostate cancer: 1999 patterns of care survey for prostate cancer. Int J Radiat Oncol Biol Phys 2004;59:1053–61.
Zietman AL, DeSilvio ML, Slater JD, et al. Comparison of conventional-dose vs. high-dose conformal radiation therapy in clinically localized adenocarcinoma of the prostate: a randomized controlled trial. JAMA 2005;294:1233–9.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Goldner, G., Bombosch, V., Geinitz, H. et al. Moderate risk-adapted dose escalation with three-dimensional conformal radiotherapy of localized prostate cancer from 70 to 74 Gy. Strahlenther Onkol 185, 94–100 (2009). https://doi.org/10.1007/s00066-009-1970-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00066-009-1970-3