Purpose:
To analyze the reliability of different methods used in evaluating the risk of late rectal toxicity.
Patients and Methods:
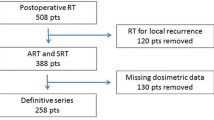
The treatment plans of 57 patients treated at the authors' institute between September 1999 and September 2000 for localized prostate cancer using three-dimensional conformal radiotherapy (3D-CRT) were analyzed retrospectively. The expected rate of late rectal toxicity was analyzed (a) by means of the dose-volume histogram (DVH) constraints; (b) by calculating the normal-tissue complication probability (NTCP) using the Lyman-Kutcher-Burman (LKB) model with the radiobiological parameters of either Emami (1991; for toxicity of grade ≥ 2) or Rancati (2004; for toxicity of grade ≥ 2 and ≥ 3). Patients were divided into high-/low-risk (HR/LR) groups and the results were compared to the clinical outcome.
Results:
(a) The HR percentages were 24% and 5% for radical and postsurgical 3D-CRT, respectively. When applying high-dose constraints only, HR percentages were 18% and 5%, respectively. (b) In the case of the NTCP (grade ≥ 2), Emami (1991) HR rates were 16% and 11%, and Rancati (2004) HR rates 29% and 11%, for radical and postsurgical treatment, respectively. Only one case with higher-grade toxicity was found. The reported clinical toxicity was 17.8% and 6.7% for grade ≥ 2 toxicity, and 3.7% and 0.7% for grade ≥ 3 toxicity, for radical and postsurgical treatment, respectively.
Conclusion:
This study demonstrated that there is an agreement between the toxicity rate evaluated by DVH constraints and by the LKB model and the clinical outcome. In this case, the use of the LKB model can be as reliable as the use of DVH constraints.
Ziel:
Analyse der Zuverlässigkeit verschiedener Methoden zur Bewertung des Risikos rektaler Spättoxizität.
Patienten und Methodik:
Die Bestrahlungspläne von 57 Patienten mit lokal begrenztem Prostatakarzinom, die sich von September 1999 bis September 2000 am Institut der Autoren einer dreidimensionalen konformalen Strahlentherapie (3D-CRT) unterzogen, wurden retrospektiv analysiert. Die erwartete rektale Spättoxizität wurde anhand von a) Dosis-Volumen-Histogramm-(DVH-)Beschränkungen und b) Wahrscheinlichkeitsberechnungen von Normalgewebskomplikationen mit dem Lyman-Kutcher-Burman-(LKB-)Modell sowie mit den radiobiologischen Parametern nach Emami et al. (1991; für Toxizitätsgrad ≥ 2) oder Rancati et al. (2004; für Toxizitätsgrade ≥ 2 and ≥ 3) untersucht. Die Patienten wurden in je eine Gruppe mit hohem und geringem Risiko (HR/LR) unterteilt, und die Ergebnisse wurden mit den klinischen Daten verglichen.
Ergebnisse:
a) Der HR-Anteil lag für die radikale 3D-CRT bei 24% und für die 3D-CRT nach Resektion bei 5%. Bei alleiniger Anwendung der Hochdosiseinschränkungen betrug der HR-Anteil 18% bzw. 5%. b) Bei Einsatz der NTCP (Grad ≥ 2) ergaben sich HR-Raten nach Emami et al. (1991) von 16% und 11% sowie nach Rancati et al. (2004) von 29% und 11% für die radikale und die postoperative Bestrahlung. Nur in einem Fall fand sich eine höhergradige Toxizität. Die berichtete klinische Toxizitätsrate betrug 17,8% bzw. 6,7% (Toxizitätsgrad ≥ 2) sowie 3,7% bzw. 0,7% (Toxizitätsgrad ≥ 3) für die radikale bzw. postoperative Behandlung.
Schlussfolgerung:
Die Studie zeigt, dass es eine Übereinstimmung zwischen der mit DVH-Beschränkungen und dem LKB-Modell ermittelten Toxizitätsrate und den klinischen Ergebnissen gibt. In diesem Fall kann sich die Verwendung des LKB-Modells als ebenso zuverlässig wie die DVH-Beschränkungen erweisen.
Similar content being viewed by others
References
Aus G, Abbou CC, Bolla M, et al. EAU guidelines on prostate cancer. Eur Urol 2005;48:546–51.
Boehmer D, Kuczer D, Badakhshi H, et al. Influence of organ at risk definition on rectal dose-volume histograms in patients with prostate cancer undergoing external-beam radiotherapy. Strahlenther Onkol 2006;182:277–82.
Boehmer D, Maingon P, Poortmans P, et al. on behalf of the EORTC Radiation Oncology Group. Guidelines for primary radiotherapy of patients with prostate cancer. Radiother Oncol 2006;79:259–69.
Boersma LJ, van den Brink M, Bruce AM, et al. Estimation of the incidence of late bladder and rectum complication after high-dose (70-78 Gy) conformal radiotherapy for prostate cancer, using dose-volume histograms. Int J Radiat Oncol Biol Phys 1998;41:83–92.
Bovi J, Qi XS, White J, et al. Comparison of three accelerated partial breast irradiation techniques: treatment effectiveness based upon biological models. Radiother Oncol 1997;44:226–32.
Cambria R, Orecchia R. Conformal radiotherapy optimization with TCP and NTCP models: pelvis and abdomen. Physica Medica 2001;XVII:Suppl 2: 103–11.
Cheung R, Tucker SL, Ye JS, et al. Characterization of rectal normal tissue complication probability after high dose external beam radiotherapy for prostate cancer. Int J Radiat Oncol Biol Phys 2004;58:1513–9.
Cozzi L, Buffa FM, Fogliata A. Comparative analysis of dose volume histogram reduction algorithms for normal tissue complication probability calculations. Acta Oncol 2000;39:165–71.
Cox JD, Stetz J, Pajak TF. Toxicity criteria of the Radiation Therapy Oncology Group (RTOG) and the European Organization for Research and Treatment of Cancer (EORTC). Int J Radiat Oncol Biol Phys 1995;31:1341–6.
Dale E, Olsen DR, Fosså SD. Normal tissue complication probabilities correlated with late effects in rectum after prostate conformal radiotherapy. Int J Radiat Oncol Biol Phys 1999;43:385–91.
Deasy JO, Niemierko A, Herbert D, et al. AAPM/NIH. Methodological issues in radiation dose-volume outcome analyses: summary of a joint AAPM/NIH workshop. Med Phys 2002;29:2109–27.
De Meerleer GO, Vakaet LA, De Gersem WR, et al. Radiotherapy of prostate cancer with or without intensity modulated beams: a planning comparison. Int J Radiat Oncol Biol Phys 2000;47:639–48.
Dörr W, Jaal J, Zips D. Prostate cancer: biological dose considerations and constraints in tele- and brachytherapy. Strahlenther Onkol 2007;183:Special Issue 2:14–5.
Emami B, Lyman J, Brown A, et al. Tolerance of normal tissue to therapeutic irradiation. Int J Radiat Oncol Biol Phys 1991;21:109–22.
Fiorino C, Cozzarini C, Vavassori V, et al. Relationship between DVHs and late rectal bleeding after radiotherapy for prostate cancer: analysis of large group of patients pooled from three institutions. Radiother Oncol 2002;64:1–12.
Georg D, Kroupa B, Georg P, et al. Inverse planning - a comparative intersystem and interpatient constraint study. Strahlenther Onkol 2006;182:473–80.
Greco C, Mazzetta C, Cattani C, et al. Finding dose-volume constraints to reduce late rectal toxicity following 3D-conformal radiotherapy (3D-CRT) of prostate cancer. Radiother Oncol 2003;69:215–22.
Guckenberger M, Flentje M. Intensity-modulated radiotherapy (IMRT) of localized prostate cancer. Strahlenther Onkol 2006;183:57–62.
Guckenberger M, Pohl F, Baier K, et al. Influence of rectum delineation (rectal volume vs. rectal wall) on IMRT treatment planning of the prostate. Strahlenther Onkol 2006;182:721–6.
Gulliford SL, Webb S, Rowbottom CG, et al. Use of artificial neural networks to predict biological outcomes for patients receiving radical radiotherapy of the prostate. Radiother Oncol 2004;71:3–12.
Hartford AC, Niemierko A, Adams JA, et al. Conformal irradiation of the prostate: estimating long-term rectal bleeding risk using dose-volume histograms. Int J Radiat Oncol Biol Phys 1996;36:721–30.
Hille A, Töws N, Schmidberger H, et al. A prospective three-dimensional analysis about the impact of differences in the clinical target volume in prostate cancer irradiation on normal-tissue exposure. Strahlenther Onkol 2005;181:789–95.
Holzapfel K, Müller SA, Seidl C, et al. Effects of irradiation on the [methyl- 3H]choline uptake in the human prostate cancer cell lines LNCaP and PC3. Strahlenther Onkol 2008;184:319–24.
Jackson A, Skwarchuk MW, Zelefsky MJ, et al. Late rectal bleeding after conformal radiotherapy of prostate cancer. II. Volume effects and dose-volume histograms. Int J Radiat Oncol Biol Phys 2001;49:685–98.
Jain NL, Kahn MG, Drzymala RE, et al. Objective evaluation of 3D radiation treatment plans: a decision-analytic tool incorporating treatment preferences of radiation oncologists. Int J Radiat Oncol Biol Phys 1993;26:321–33.
Jereczek-Fossa BA, Cattani F, Garibaldi C, et al. Transabdominal ultrasonography, computed tomography and electronic portal imaging for 3-dimensional conformal radiotherapy for prostate cancer. Strahlenther Onkol 2007;183:610–6.
Jereczek-Fossa BA, Orecchia R. Evidence-based radiation oncology: definitive,adjuvant and salvage radiotherapy for non-metastatic prostate cancer. Radiother Oncol 2007;84:197–215.
Jereczek-Fossa BA, Zerini D, Vavassori A, et al. Sooner or later? Outcome analysis of 431 prostate cancer patients treated with postoperative or salvage radiotherapy. Int J Radiat Oncol Biol Phys 2009;74:115–25.
Kutcher GJ, Burman C. Calculation of the complication probability factors for the non-uniform normal tissue irradiation: the effective volume method. Int J Radiat Oncol Biol Phys 1989;16:1623–30.
Kutcher GJ, Burman C, Brewster L, et al. Histogram reduction method for calculating complication probabilities for three-dimensional treatment planning evaluations. Int J Radiat Oncol Biol Phys 1991;21:137–46.
Landoni V, Saracino B, Marzi S, et al. A study of the effect of set-up errors and organ motion on prostate cancer treatment with IMRT. Int J Radiat Oncol Biol Phys 2006;65:587–94.
Lawton CA, Won M, Pilepich MV, et al. Long-term treatment sequelae following external beam irradiation for adenocarcinoma of the prostate: analysis of RTOG studies 7506 and 7706. Int J Radiat Oncol Biol Phys 1991;21:935–9.
Lebesque JV, Bruce AM, Kroes AP, et al. Variation in volume, dose-volume histograms, and estimated normal tissue complication probabilities of rectum and bladder during conformal radiotherapy of T3 prostate cancer. Int J Radiat Oncol Biol Phys 1995;33:1109–19.
Luxton G, Hancock SL, Boyer AL. Dosimetry and radiobiologic model comparison of IMRT and 3D conformal radiotherapy in treatment of carcinoma of the prostate. Int J Radiat Oncol Biol Phys 2004;59:267–84.
Lyman JT. Complication probability as assessed from dose-volume histograms. Radiat Res Suppl 1985;8:S13–9.
Marzi S, Arcangeli G, Saracino B, et al. Relationships between rectal wall dose-volume constraints and radiobiologic indices of toxicity for patients with prostate cancer. Int J Radiat Oncol Biol Phys 2007;68:41–9.
Mavroidis P, al-Abany M, Helgason AR, et al. Dose-response relations for anal sphincter regarding fecal leakage and blood or phlegm in stools after radiotherapy for prostate cancer. Radiobiological study of 65 consecutive patients. Strahlenther Onkol 2005;181:293–306.
Neal AJ, Oldham M, Dearnaley DP. Comparison of treatment techniques for conformal radiotherapy of the prostate cancer using dose-volume histograms and normal tissue complication probabilities. Radiother Oncol 1995;37:29–34.
Oldham M, Neal A, Webb S. A comparison of conventional “forward planning” with inverse planning for 3D conformal radiotherapy of the prostate. Radiother Oncol 1995;35:248–62.
Peeters STH, Hoogeman MS, Heemsbergen, et al. Rectal bleeding, fecal incontinence, and high stool frequency after conformal radiotherapy for prostate cancer: normal tissue complication probability modeling. Int J Radiat Oncol Biol Phys 2006;66:11–9.
Ragazzi G, Mangili P, Fiorino C, et al. Variations of tumor control probability and rectum complication probabilities due to random set-up errors during conformal radiation therapy of prostate cancer. Radiother Oncol 1997;44:259–63.
Rancati T, Fiorino C, Gagliardi G, et al. Fitting late rectal bleeding data using different NTCP models: results from an Italian multi-centric study (AIROPROS0101). Radiother Oncol 2004;73:21–32.
Roach M, Pickett B, Weil M, et al. The “critical volume tolerance method” for estimating the limits of dose escalation during three-dimensional conformal radiotherapy for prostate cancer. Int J Radiat Oncol Biol Phys 1996;35:1019–28.
Sanchez-Nieto B, Nahum AE, Dearnaley DP. Individualization of dose prescription based on normal-tissue dose-volume and radiosensitivity data. Int J Radiat Oncol Biol Phys 2001;49:487–99.
Sanguineti G, Cavey ML, Endres EJ, et al. Does treatment of the pelvic nodes with IMRT increase late rectal toxicity over conformal prostate-only radiotherapy to 76 Gy? Strahlenther Onkol 2006;182:543–9.
Simpson JR, Purdy JA, Manolis JM, et al. Three dimensional treatment planning considerations for prostate cancer. Int J Radiat Oncol Biol Phys 1991;21:243–82.
Skwarchuk MW, Jackson A, Zelefsky MJ, et al. Late rectal toxicity after conformal radiotherapy of prostate cancer (I): multivariate analysis and dose-response. Int J Radiat Oncol Biol Phys 2004;7:103–13.
Tucker SL, Zhang M, Dong L, et al. Cluster model analysis of late rectal bleeding after IMRT of prostate cancer: a case-control study. Int J Radiat Oncol Biol Phys 2006;64:1255–64.
Vargas C, Martinez A, Kestin LL, et al. Dose-volume analysis of predictors for chronic rectal toxicity after treatment of prostate cancer with adaptive image-guided radiotherapy. Int J Radiat Oncol Biol Phys 2005;62:1297–308.
Webb S. Converting 3D dose to biological outcomes: tumour control probability (TCP) and normal-tissue-complication probability (NTCP). In: Mould RF, Orton CG, Spaan JA, et al., eds. The physics of conformal radiotherapy. Bristol-Philadelphia: IOP, CRC Press, 1997:258–85
Weiss W, Horninger W, Forthuber BC, et al. Single-institution results of primary external-beam radiation for the treatment of T1-T3 prostate cancer. Strahlenther Onkol 2007;183:321–6.
Witte MG, van der Geer J, Schneider C, et al. IMRT optimization including random and systematic geometric errors based on the expectation of TCP and NTCP. Med Phys 2007;34:3544–55.
Zaider M, Amols HI. Practical considerations in using calculated healthy-tissue complication probabilities for the treatment-plan optimization. Int J Radiat Oncol Biol Phys 1999;44:439–47.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Cambria, R., Jereczek-Fossa, B.A., Cattani, F. et al. Evaluation of late rectal toxicity after conformal radiotherapy for prostate cancer. Strahlenther Onkol 185, 384–389 (2009). https://doi.org/10.1007/s00066-009-1933-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00066-009-1933-8