Abstract
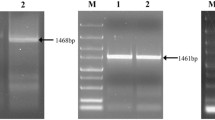
Plant cystatins play crucial roles in the process of plant defense against herbivorous insects. A cDNA clone, designated CsCPI2, was isolated from Camellia sinensis cv. Longjing 43 using 5′-/3′-RACE extension. The full-length cDNA gene is 618 bp in size, encodes 205 amino acid residues, and has a deduced molecular weight of 23.07 kDa. CsCPI2 was different from the already published CsCPI1, as the two cDNA sequences share 69.31% and 60.4% pairwise identity at the nucleic acid and amino acid levels, respectively. The CsCPI2–MBP fusion protein was over-expressed in Escherichia coli. We found that the inhibitory effect of CsCPI2–MBP on ficin was 1.17, 3.46, and 3.73 times the effect on papain, chymopapain, and bromelain, respectively. The highest inhibitory activity of CsCPI2–MBP was found at pH 7 at 40 °C, and CsCPI2–MBP was stable at temperature below 60 °C. Moreover, CsCPI2–MBP was found to be more responsive to acidic environment than to alkaline one. Higher cystatin inhibitory activity was found in the tea leaves infested by Myllocerinus aurolineatus than that in intact healthy leaves. Finally, we found that CsCPI2 was a competitive inhibitor of the cysteine proteinases in tea weevil’s gut, and leaves treated with CsCPI2–MBP incurred compensatory damage, and the weight gain of tea weevils fed on CsCPI2–MBP treated leaves was significantly higher than that of MBP treated leaves, but did not cause mortality. Taken together, our research highlights potential defensive proteins for future tea engineering approaches, but more in vivo experiments are needed to confirm their effectiveness.







Similar content being viewed by others
References
Abdeen A, Virgos A, Olivella E et al (2005) Multiple insect resistance in transgenic tomato plants over-expressing two families of plant proteinase inhibitors. Plant Mol Biol 57:189–202
Abe M, Arai S (1991) Some properties of a cysteine proteinase inhibitor from corn endosperm. Agric Biol Chem 55:2417–2418
Ahn JE, Salzman RA, Braunagel SC et al (2004) Functional roles of specific bruchid protease isoforms in adaptation to a soybean protease inhibitor. Insect Mol Biol 13:649–657
Bangrak P, Chotigeat W (2011) Molecular cloning and biochemical characterization of a novel cystatin from Hevea rubber latex. Plant Physiol Biochem 49:244–250
Barrett AJ, Fritz H, Grubb A et al (1986) Nomenclature and classification of the proteins homologous with the cysteine-proteinase inhibitor chicken cystatin. Biochem J 236:312
Benchabane M, Schlüter U, Vorster J et al (2010) Plant cystatins. Biochemie 92:1657–1666
Bode RF, Halitschke R, Kessler A (2013) Herbivore damage-induced production and specific anti-digestive function of serine and cysteine protease inhibitors in tall goldenrod, Solidago altissima L. (Asteraceae). Planta 237:1287–1296
Botelhojúnior S, Machado OL, Fernandes KV et al (2014) Defense response in non-genomic model species: methyl jasmonate exposure reveals the passion fruit leaves’ ability to assemble a cocktail of functionally diversified Kunitz-type trypsin inhibitors and recruit two of them against papain. Planta 240:345–356
Botella MA, Xu Y, Prabha TN et al (1996) Differential expression of soybean cysteine proteinase inhibitor genes during development and in response to wounding and methyl jasmonate. Plant Physiol 112:1201–1210
Bouchard E, Cloutier C, Michaud D (2003) Oryzacystatin I expressed in transgenic potato induces digestive compensation in an insect natural predator via its herbivorous prey feeding on the plant. Mol Ecol 12:2439–2446
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72(1–2):248–254
Broadway RM (1997) Dietary regulation of serine proteinases that are resistant to serine proteinase inhibitors. J Insect Physiol 43:855–874
Cai XM, Sun XL, Dong WX et al (2014) Herbivore species, infestation time, and herbivore density affect induced volatiles in tea plants. Chemoecology 24:1–14
Carrillo L, Martinez M, Alvarez-Alfageme F et al (2011) A barley cysteine-proteinase inhibitor reduces the performance of two aphid species in artificial diets and transgenic Arabidopsis plants. Transgenic Res 20:305–319
Cingel A, Savić J, Ćosić T et al (2014) Pyramiding rice cystatin OCI and OCII genes in transgenic potato (Solanum tuberosum L.) for resistance to Colorado potato beetle (Leptinotarsa decemlineata Say). Euphytica 198:425–438
Cloutier C, Jean C, Fournier M et al (2010) Adult Colorado potato beetles, Leptinotarsa decemlineata, compensate for nutritional stress on oryzacystatin I-transgenic potato plants by hypertrophic behavior and over-production of insensitive proteases. Arch Insect Biochem Physiol 44:69–81
De Leo F, Bonade-Bottino MA, Ceci LR et al (1998) Opposite effects on Spodoptera littoralis larvae of high expression level of a trypsin proteinase inhibitor in transgenic plants. Plant Physiol 118:997–1004
Gaddour K, Vicentecarbajosa J, Lara P et al (2001) A constitutive cystatin-encoding gene from barley (Icy) responds differentially to abiotic stimuli. Plant Mol Biol 45:599–608
Gatehouse JA (2011) Prospects for using proteinase inhibitors to protect transgenic plants against attack by herbivorous insects. Curr Protein Pept Sci 12:409–416
Girard C, Rivard D, Kiggundu A et al (2007) A multicomponent, elicitor-inducible cystatin complex in tomato, Solanum lycopersicum. New Phytol 173:841–851
Green TR, Ryan CA (1972) Wound-induced proteinase inhibitor in plant leaves: a possible defense mechanism against insects. Science 175:776–777
Grima R, Walter NG, Schnell S (2014) Single-molecule enzymology à la Michaelis-Menten. FEBS J 281:518–530
Ievleva EV, Rudenskaya YA, Zimacheva AV et al (1995) Cystatin from barley seeds. Biochemistry (Moscow) 60:1237–1240
Ji R, Ye W, Chen H et al (2017) A salivary endo-β-1,4-glucanase acts as an effector that enables the brown planthopper to feed on rice. Plant Physiol 173:1920–1932
Johnson KS, Rabosky D (2000) Phylogenetic distribution of cysteine proteinases in beetles: evidence for an evolutionary shift to an alkaline digestive strategy in cerambycidae. Comp Biochem Physiol B: Biochem Mol Biol 126:609–619
Jongsma MA, Bolter C (1997) The adaptation of insects to plant protease inhibitors. J Insect Physiol 43:885–895
Kim E, Kim Y, Yeam I et al (2016) Transgenic expression of a viral cystatin gene cpbv-cst1 in tobacco confers insect resistance. Environ Entomol 45:1322–1331
Lepelley M, Amor MB, Martineau N et al (2012) Coffee cysteine proteinases and related inhibitors with high expression during grain maturation and germination. BMC Plant Biol 12:31–48
Liang C, Brookhart GL, Feng GH et al (1991) Inhibition of digestive proteinases of stored grain Coleoptera by oryzacystatin, a cysteine proteinase inhibitor from rice seed. FEBS Lett 278:139–142
Liang J, Wang Y, Ding G et al (2015) Biotic stress-induced expression of mulberry cystatins and identification of cystatin exhibiting stability to silkworm gut proteinases. Planta 242:1139–1151
Liu S, Han B (2010) Differential expression pattern of an acidic 9/13-lipoxygenase in flower opening and senescence and in leaf response to phloem feeders in the tea plant. BMC Plant Biol 10:228–243
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 25:402–408
Ma J, Tanaka KF, Shimizu T et al (2011) Microglial cystatin f expression is a sensitive indicator for ongoing demyelination with concurrent remyelination. J Neurosci Res 89:639–649
Margis R, Reis EM, Villeret V (1998) Structural and phylogenetic relationships among plant and animal cystatins. Arch Biochem Biophys 359:24–30
Martinez M, Diaz-Mendoza M, Carrillo L et al (2007) Carboxy terminal extended phytocystatins are bifunctional inhibitors of papain and legumain cysteine proteinases. FEBS Lett 581:2914–2918
Martinez M, Cambra I, Carrillo L (2009) Characterization of the entire cystatin gene family in barley and their target cathepsin l-like cysteine-proteases, partners in the horde in mobilization during seed germination. Plant Physiol 151:1531–1545
Martinez M, Santamaria ME, Diaz-Mendoza M et al (2016) Phytocystatins: defense proteins against phytophagous insects and acari. Int J Mol Sci 17:1747
Michaelis L, Menten ML (1913) Die Kinetik der Invertinwirkung. Biochem Z 49:333–369
Michaud D, Bernier-Vadnais N, Overney S et al (1995) Constitutive expression of digestive cysteine proteinase forms during development of the colorado potato beetle, Leptinotarsa decemlineata Say (Coleoptera: Chrysomelidae). Insect Biochem Mol Biol 25(9):1041–1048
Nissen MS, Kumar GN, Youn B et al (2009) Characterization of Solanum tuberosum multicystatin and its structural comparison with other cystatins. Plant Cell 21:861–875
Oppert B, Morgan TD, Hartzer K, Kramer KJ (2005) Compensatory proteolytic responses to dietary proteinase inhibitors in the red flour beetle, Tribolium castaneum (Coleoptera: Tenebrionidae). Comp Biochem Physiol C: Toxicol Pharmacol 140:53–58
Otto HH, Schirmeister T (1997) Abstract: cysteine proteases and their inhibitors. Cheminform 28:133–172
Pan J (2014) Toxic effects of resistance associated proteins (Cs-cystatin, Cs-Rip) of tea on Empoasca vitis. Master’s thesis. University of Xiamen
Papolu PK, Dutta TK, Nidhi T et al (2016) Expression of a cystatin transgene in eggplant provides resistance to root-knot nematode, Meloidogyne incognita. Front Plant Sci 7:1122
Qin D, Zhang L, Xiao Q et al (2015) Clarification of the identity of the tea green leafhopper based on morphological comparison between Chinese and Japanese specimens. PLoS ONE 10:e0139202
Rawlings ND, Barrett AJ (1990) Evolution of proteins of the cystatin superfamily. J Mol Evol 30:60–71
Rawlings ND, Tolle DP, Barrett AJ (2004) Evolutionary families of peptidase inhibitors. Biochem J 378:705–716
Ribeiro APO, Pereira EJG, Galvan TL et al (2006) Effect of eggplant transformed with oryzacystatin gene on Myzus persicae and Macrosiphum euphorbiae. J Appl Entomol 130:84–90
Schneider VK, Soares-Costa A, Chakravarthi M et al (2017) Transgenic sugarcane overexpressing CaneCPI-1 negatively affects the growth and development of the sugarcane weevil Sphenophorus levis. Plant Cell Rep 36:193–201
Shamim M, Khan NA, Singh KN (2011) Inhibition of midgut protease of yellow stem borer (Scirpophaga incertulas) by cysteine protease-like inhibitor from mature jackfruit (Artocarpus heterophyllus) seed. Acta Physiol Plant 33:2249–2257
Singh M, Bhogal D, Goel A et al (2011) Cloning, in silico characterization and interaction of cysteine protease and cystatin for establishing their role in early blight disease in tomato. J Plant Biochem Biotechnol 20:110–117
Stubbs MT, Laber B, Bode W et al (1990) The refined 2.4a x-ray crystal structure of recombinant human stefin B in complex with the cysteine proteinase papain: a novel type of proteinase inhibitor interaction. EMBO J 9:1939–1947
Sun XL, Wang GC, Cai XM et al (2010) The tea weevil, Myllocerinus aurolineatus, is attracted to volatiles induced by conspecifics. J Chem Ecol 36:388–395
Sun XL, Wang GC, Gao Y et al (2012) Screening and field evaluation of synthetic volatile blends attractive to adults of the tea weevil, Myllocerinus aurolineatus. Chemoecology 22:229–237
Sun XL, Wang GC, Gao Y et al (2014) Volatiles emitted from tea plants infested by Ectropis obliqua larvae are attractive to conspecific moths. J Chem Ecol 40:1080–1089
Walsh TA, Strickland JA (1993) Proteolysis of the 85-kilodalton crystalline cysteine proteinase inhibitor from potato releases functional cystatin domains. Plant Physiol. 103:1227–1234
Wang ZX, Li YY, Jiang CJ et al (2005) Molecular cloning and sequence analysis on cDNA of cystatin gene from tea leaves. J Tea Sci 25:177–182
Wang W, Zhao P, Zhou XM et al (2015) Genome-wide identification and characterization of cystatin family genes in rice (Oryza sativa L.). Plant Cell Rep 34:1579–1592
Winterer J, Bergelson J (2001) Diamondback moth compensatory consumption of protease inhibitor transformed plants. Mol Ecol 10:1069–1074
Xin ZJ, Zhang LP, Zhang ZQ et al (2014) A tea hydroperoxide lyase gene, CsiHPL1, regulates tomato defense response against Prodenia Litura (Fabricius) and Alternaria Alternata f. sp. Lycopersici by modulating green leaf volatiles (GLVs) release and jasmonic acid (JA) gene expression. Plant Mol Biol Rep 32:62–69
Xin ZJ, Li XW, Li JC et al (2016) Application of chemical elicitor (Z)-3-hexenol enhances direct and indirect plant defenses against tea geometrid Ectropis obliqua. Biocontrol 61:1–12
Xin ZJ, Zhang J, Ge LG et al (2017) A putative 12-oxophytodienoate reductase gene CsOPR3 from Camellia sinensis is involved in wound and herbivore infestation responses. Gene 615:18–24
Ye GY, Xiao Q, Chen M et al (2014) Tea: biological control of insect and mite pests in China. Biol Control 68:73–91
Zhao Y, Botella MA, Subramanian L et al (1996) Two wound-inducible soybean cysteine proteinase inhibitors have greater insect digestive proteinase inhibitory activities than a constitutive homolog. Plant Physiol 111:1299–1306
Zhusalzman K, Zeng R (2015) Insect response to plant defensive protease inhibitors. Annu Rev Entomol 60:233–252
Author information
Authors and Affiliations
Contributions
The study was funded by the National Natural Science Foundation of China (31471784; 31772180), Central Public-interest Scientific Institution Basal Research Fund (1610212017017; 1610212018004), and Zhejiang Provincial Natural Science Foundation of China (LQ18C160008).
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Handling Editor: Günther Raspotnig.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Zhang, X., Ran, W., Liu, F. et al. Cloning, expression and enzymatic characterization of a cystatin gene involved in herbivore defense in tea plant (Camellia sinensis). Chemoecology 30, 233–244 (2020). https://doi.org/10.1007/s00049-020-00312-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00049-020-00312-6