Abstract
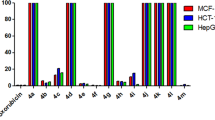
A set of 2-amino-4-aryl-4H-pyrano[3,2-h]quinoline-3-carbonitrile derivatives were prepared via a one-pot, three-component condensation reaction between the substituted hydroxyquinoline derivatives, some aryl and/or hetaryl aldehydes, and malononitrile in an ethanol/piperidine solution in a microwave irradiation environment. The structure of the prepared compounds was instituted on the foundations of their spectral data: IR, 1H NMR, 13C NMR, and MS. Four human cancer cell lines, MCF-7, HCT-116, HepG-2, and A549 were utilized to evaluate the antiproliferative properties of the target compounds in comparison to the positive controls, Vinblastine and Colchicine using the MTT viability assay. The cell cycle arrest behavior, detected by propidium iodide as well as the apoptosis induction, which was monitored by the flow cytometer, using the Annexin V-FITC kits, was investigated. The results illustrated that the potent cytotoxic compounds induce cell cycle arrest at the G2/M phases and trigger apoptosis in the different tested cancer cells. Finally, the structure−activity relationship (SAR) study showcases the substitution of some specific groups at the 4-, 6-, and 9-positions in the prepared 2-amino-4H-pyrano[3,2-h]quinoline derivatives, which indicates that the lipophilicity manipulates the ability of these moieties against the diverse cell lines.






Similar content being viewed by others
References
Al-Ghamdi AM, Abd EL-Wahab AHF, Mohamed HM, El-Agrody AM (2012) Synthesis and antitumor activities of 4H-pyrano[3,2-h]quinoline-3-carbonitrile, 7H-pyrimido[4′,5′:6,5] pyrano[3,2-h]quinoline, and 14H-Pyrimido[4′,5′:6,5]pyrano[3,2-h][1,2,4]triazolo[1,5-c]quinoline derivative. Lett Drug Des Discov 9:459–470
Balamurugan K, Jeyachandran V, Perumal S, Manjashetty TH, Yogeeswari P, Sriram D (2010) A microwave-assisted, facile, regioselective Friedländer synthesis and antitubercular evaluation of 2,9-diaryl-2,3-dihydrothieno[3,2-b]quinolones. Eur J Med Chem 45:682–688
Batt DG, Petraitis JJ, Sherk SR, Copeland RA, Dowling RL, Taylor TL, Jones EA, Magolda RL, Jafee BD (1998) Heteroatom- and carbon-linked biphenyl analogs of Brequinar as immunosuppressive agents. Bioorg Med Chem Lett 8:1745–1750
Cairns H, Cox D, Gould K, Ingall A, Suschitzky J (1985) New antiallergic pyrano[3,2-g] quinoline-2, 8-dicarboxylic acids with potential for the topical treatment of asthma. J Med Chem 28:1832–1834
Chang FS, Chen W, Wang C, Tzeng CC, Chen YL (2010) Synthesis and antiproliferative evaluations of certain 2-phenylvinylquinoline (2-styrylquinoline) and 2-furanylvinylquinoline derivatives. Bioorg Med Chem 18:124–133
Chen IS, Tsai IL, Teng C, Chen JJ, Chang YA, Ko FN, Lu MC, Pezzuto JM (1997) Pyranoquinoline alkaloids from Zanthoxylum simulans. Phytochemistry 46:525–529
Chen IS, Wu SJ, Tsai IL, Wu TS, Pezzuto JM, Lu MC, Chai H, Suh N, Teng CMJ (1994) Chemical and bioactive constituents from Zanthoxylum simulans. Nat Prod 57:1206–1211
Chen J, Chen P, Liao C, Huang S, Chen I (2007) New phenylpropenoids, bis (1-phenylethyl) phenols, bisquinolinone alkaloid, and anti-inflammatory constituents from Zanthoxylum integrifoliolum. J Nat Prod 70:1444–1448
Demirci F, Bas¸er KHC (2002) Bioassay techniques for drug development By Atta-ur-Rahman, M. Iqbal Choudhary (HEJRIC, University of Karachi, Pakistan), William J. Thomsen (Areana Pharmaceuticals, San Diego, CA). Harwood Academic Publishers, Amsterdam, the Netherlands. xii+223pp. J Nat Prod 65:1086–1087
Doube D, Blouin M, Brideau C, Chan C, Desmarais C, Ethier D, Falgueyret JP, Friesen RW, Girard M, Girard Y, Guay J, Tagari P, Young RN (1998) Quinolines as potent 5-lipoxygenase inhibitors: synthesis and biological profile of L-746,530. Bioorg Med Chem Lett 8:1255–1260
Edmont D, Rocher R, Plisson C, Chenault J (2000) Synthesis and evaluation of quinoline carboxyguanidines as antidiabetic agents. Bioorg Med Chem Lett 10:1831–1834
El-Agrody AM, Abd-Rabboh HSM, Al-Ghamdi AM (2013) Synthesis, antitumor activity, and structure-activity relationship of some 4H-pyrano[3,2-h]quinolone and 7H-pyrimido[4′,5′:6,5]pyrano[3,2-h]quinolone derivatives. Med Chem Res 22:1339–1355
El-Agrody AM, Al-Ghamdi AM (2011) Synthesis of certain novel 4H-pyrano[3,2-h] quinoline derivatives ARKIVOC xi_134–146
El-Agrody AM, Khattab ESAEH, Fouda AM, Al-Ghamdi AM (2012) Synthesis and antitumor activities of certain novel 2-amino-9-(4-halostyryl)-4H-pyrano[3,2-h]quinoline derivatives. Med Chem Res 12:4200–4213
Fouda AM (2017) Halogenated 2-amino-4H-pyrano[3,2-h]quinoline-3-carbonitriles as antitumor agents and structure-activity relationships of the 4-, 6-, and 9-positions. Med Chem Res 26:302–313
Hammouda MAA, EL-Hag FA-AA, El-Serwy WS, El-Manawaty MA (2015) Synthesis and characterization of new fused 4H-Pyranquinoline carbonitrile derivatives with anticipated antitumor biological activity. RJPBCS 6:200–208
Hassanin HM, Ibrahim MA, Alnamer YA-S (2012) Synthesis and antimicrobial activity of some novel 4-hydroxyquinolin-2(1H)-ones and pyrano[3,2-c]quinolinones from 3-(1-ethy1-4-hydroxy-2-oxo-1,2-dihydroquinolin-3-yl)-3-oxopropanoic acid. Turk J Chem 36:682–699
Isaka M, Tanticharoen M, Kongsaeree P, Thebtaranonth Y (2001) Structures of cordypyridones A−D, antimalarial N-hydroxy- and N-methoxy-2-pyridones from the insect pathogenic fungus Cordyceps nipponica. J Org Chem 66:4803–4808
Kamperdick C, Van NH, Van Sung T, Adam GB (1999) Bisquinolinone alkaloids from Melicope ptelefolia. Phytochemistry 50:177–181
Kappe CO (2004) Controlled microwave heating in modern organic synthesis. Angew Chem Int Ed 43:6250–6284
Kaur K, Jain M, Kaur T, Jain R (2009) Antimalarials from nature. Bioorg Med Chem 17:3229–3256
Kidwai M, Saxena S, Khan MKR, Thukra SS (2005) Aqua mediated synthesis of substituted 2-amino-4H-chromenes and in vitro study as antibacterial agents. Bioorg Med Chem Lett 15:4295–4298
Koizumi F, Fukumitsu N, Zhao J, Chanklan R, Miyakawa T, Kawahara S, Iwamoto S, Suzuki M, Kakita S, Rahayu E, Hosokawa S, Tatsuta K (2007) YCM1008A, a novel Ca 2+-signaling inhibitor, produced by Fusarium sp. YCM1008. J Antibiot 60:455–458
Maalej E, Chabchoub F, Oset-Gasque MJ, Esquivias-Pérez M, González MP, Monjas L, Pérez C, de los Ríos C, Rodríguez-Franco MI, Iriepa I, Moraleda I, Chioua M, Romero A, Marco-Contelles J, Samadi A (2012) Synthesis, biological assessment, and molecular modeling of racemic 7-aryl-9,10,11,12-tetrahydro-7H-benzo[7,8]chromeno[2,3-b]quinolin-8-amines as potential drugs for the treatment of Alzheimer’s disease. Eur J Med Chem 54:750–763
Magesh CJ, Makesh SV, Perumal PT (2004) Highly diastereoselective inverse electron demand (IED) Diels–Alder reaction mediated by chiral salen–AlCl complex: the first, target-oriented synthesis of pyranoquinolines as potential antibacterial agents. Bioorg Med Chem Lett 14:2035–2040
Marco JL, Martinez-Grau A (1997) Friedländer reaction on 2-amino-3-cyano-4H-pyrans: synthesis of derivatives of 4H-pyrano[2, 3-b]quinoline, new tacrine analogues. Bioorg Med Chem Lett 7:3165–3170
Mekheimer RA, Sadek KU (2009) Microwave-assisted reactions: three-component process for the synthesis of 2-amino-2-chromenes under microwave heating Chin Chem Lett 20:271–274
Mol W, Matyia M, Filip B, Wietrzyk J, Boryczka S (1984) Synthesis and antiproliferative activity in vitro of novel (2-butynyl) thioquinolines. Bioorg Med Chem 16:8136–8141
Mosmann T (1983) Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods 65:55–63
Narsinh D, Anamik S (2001) Synthesis and anti-HIV studies of some substituted pyrimidinediones, ethoxy pyrano (3,2-C) quinolines and hydrazino pyrano (3,2-C) quinolines. Ind J Pharm Sci 63:211–215
Nesterova IN, Alekseeva LM, Andreeva LM, Andreeva NI, Golovira SM, Granik VG (1995) Synthesis and study the pharmacological activity of derivatives of 5-dimethylaminopyrano-[3,2-c]quinolin-2-ones Pharm Chem J 29:111–114
Prasad P, Shobhashana PG, Patel MP (2017) An efficient synthesis of 4H-pyranoquinolinone derivatives catalyzed by a versatile organocatalyst tetra-n-butylammonium fluoride and their pharmacological screening. R Soc Open Sci 4:170764. https://doi.org/10.1098/rsos.170764.
Qu Z, Cui J, Harata-Lee Y, Aung TN, Feng Q, Raison JM (2016) Identification of candidate anti-cancer molecular mechanisms of Compound Kushen Injection using functional genomics. Oncotarget 10:66003–66019
Rahman AU, Choudhary MI, Thomsen WJ (2001) Bioassay technique for drug development. Harwood Academic Publishers, Reading, UK.
Ramesh M, Mohan PA, Shanmugam P (1984) A convenient synthesis of flindersine, atanine and their analogues. Tetrahedron 40:4041–4049
Sechi M, Rizzi G, Bacchi A, Carcelli M, Rogolino D, Pala N, Sanchez TW, Taheri L, Dayam R, Neamati N (2009) Design and synthesis of novel dihydroquinoline-3-carboxylic acids as HIV-1 integrase inhibitors. Bioorg Med Chem 17:2925–2935
Shen JK, Du HP, Yang M, Wang YG, Jin J (2009) Casticin induces leukemic cell death through apoptosis and mitotic catastrophe. Ann Hematol 88:743–752
Shi A, Nguyen TA, Battina SK, Rana S, Takemoto DJ, Chiang PK, Hua DH (2008) Synthesis and anti-breast cancer activities of substituted quinolones. Bioorg Med Chem Lett 18:3364–3368
Shi L, Wang M, Fan CA, Zhang FM, Tu YQ (2004) Microwave-promoted three-component coupling of aldehyde, alkyne, and amine via C−H activation catalyzed by copper in water. Org Lett 6:1001–1003
Siliveri S, Radhika T, Harinadha BV, Raj S, Madhava RB (2017) Synthesis and biological evaluation of pyrano[3,2-h]quinoline carbonitriles. Int J Green Pharm 11:S423–S429
Sparatore A, Basilico N, Casagrande M, Parapini S, Taramelli D, Brun R, Wittlin S, Sparatore F (2008) Antimalarial activity of novel pyrrolizidinyl derivatives of 4-aminoquinoline. Bioorg Med Chem Lett 18:3737–3740
Surpur MP, Kshirsagar S, Samant SD (2009) Exploitation of the catalytic efficacy of Mg/Al hydrotalcite for the rapid synthesis of 2-aminochromene derivatives via a multicomponent strategy in the presence of microwaves. Tetrahedron Lett 50:719–722
Thomas KD, Adhikari AV, Shetty NS (2010) Design, synthesis and antimicrobial activities of some new quinoline derivatives carrying 1,2,3-triazole moiety. Eur J Med Chem 45:3803–3810
Van Engeland M, Nieland LJW, Ramaekers FCS, Schutte B, Reutelingsperger CPM (1998) Annexin V-affinity assay: a review on an apoptosis detection system based on phosphatidylserine exposure. Cytometry 31:1–9
Vermes I, Haanen C, Steffens-Nakken H, Teutelinger C (1995) A novel assay for apoptosis: flow cytometric detection of phosphatidylserine expression on early apoptotic cells using fluorescein labeled annexin V. J Immunol Methods 184:39–51
Wabo HK, Tane P, Connolly JD, Okunji CC, Schuster B, Iwu MM (2005) Tabouensinium chloride, a novel quaternary pyranoquinoline alkaloid from Araliopsis tabouensis. Nat Prod Res 19:591–595
Wu X, Larhed M (2005) Microwave-enhanced aminocarbonylations in water. Org Lett 7:3327–3329
Acknowledgements
The authors extend their appreciation to the Deanship of Science Research at King Khalid University for funding this work through General Research Project under Grant Number (G.R.P-168-39). In addition, the authors deeply thank the Regional Center for Mycology & Biotechnology (RCMP), Al-Azhar University, Cairo, Egypt, for carrying out the antitumor study and also, Mr. Ali Y. A. Alshahrani for drawing the 1H NMR and 13C NMR spectra.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Fouda, A.M., Youssef, A.M.S., Afifi, T.H. et al. Cell cycle arrest and induction of apoptosis of newly synthesized pyranoquinoline derivatives under microwave irradiation. Med Chem Res 28, 668–680 (2019). https://doi.org/10.1007/s00044-019-02325-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00044-019-02325-5