Abstract
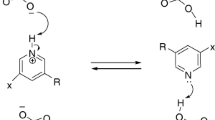
Series of novel chromonyl enaminones 1a–e and 2a–e and 3-alkylated chromones 3a–e were synthesized and evaluated in vitro as α-glucosidase inhibitors as well as antioxidant and antifungal agents. Antifungal activity was tested on strains of Candida albicans. Compounds 2a and 2d–e showed good inhibition of the α-glucosidase enzyme (IC50 = 5.5, 0.9, and 1.5 mM, respectively), their effect being better than that of 1a–e, 3a–e, and acarbose (the standard, IC50 = 7.73 ± 0.9 mM). The structure–activity relationship suggests that the phenyl group at the C-3 position of the chromone ring system and the 4-chlorophenyl group at the enaminone moiety (derivatives 2) increased the inhibition of α-glucosidase. Compounds 2a–e exhibited a slight antioxidant effect, and compounds 3a–e a moderate antifungal activity against C. albicans (IC50 70.5–83.1 µg/mL). Docking studies revealed that compounds 2 interact with the α-glucosidase residues of the binding pocket. Therefore, these chromone derivatives may be considered as potential α-glucosidase inhibitors, as well as antifungal agents against some Candida strains of yeast.






Similar content being viewed by others
References
ACD/ChemSketch (Freeware) version 14. 01 (2012). Advanced Chemistry Development, Inc., Toronto, ON, Canada
Belov P, Campanella VL, Smith AW, Priefer R (2011) Microwave-assisted methylation of phenols with DMA-DMF. Tetrahedron Lett 52:2776–2779
Berkow EL, Lockhart SR (2017) Fluconazole resistance in Candida species: a current perspective. Infect Drug Resist 10:237–245
Boonsombat J, Prachyawarakorn V, Pansanit A, Mahidol C, Ruchirawat S, Thongnest S (2017) Superbanone, a new 2-aryl-3-benzofuranone and other bioactive constituents from the tube roots of Butea superba. Chem Biodivers 14:e1700044
Brunel A-S, Guery B (2017) Multidrug resistant (or antimicrobial-resistant) pathogens—alternatives to new antibiotics. Swiss Med Wkly 147:14553–14567
Canno RD, Lamping E, Holmes AR, Niimi K, Tanabe K, Niimi M, Monk BC (2007) Candida albicans drug resistance—another way to cope with stress. Microbiology 153:3211–3217
Cevallos-Casals BA, Cisneros-Zevallos L (2003) Stoichiometric and kinetic studies of phenolic antioxidants from Anden purple corn and red-fleshed sweetpotato. J Agric Food Chem 51:3313–3319
Chin MR, Zlotkowski K, Han M, Patel S, Eliasen AM, Axelrod A, Siegel D (2015) Expedited access to vinaxanthone and chemically edited derivatives possessing neuronal regenerative effects through ynone coupling reactions. ACS Chem Neurosci 5:542–550
Chohan ZH, Rauf A, Naseer MM, Somra MA, Supuran CT (2006) Antibacterial, antifungal and cytotoxic properties of some sulfonamide-derived chromones. J Enzyme Inhib Med Chem 21:173–177
Correa C, Cruz MC, Jiménez F, Zepeda LG, Tamariz J (2008) A new synthetic route of 2-aryl- and 2-benzyl-benzofurans and their application in the total synthesis of a metabolite isolated from Dorstenia gigas. Aust J Chem 61:991–999
Csepanyi E, Szabados-Furjesi P, Kiss-Szikszai A, Frensemeier LM, Karst U, Lekli I, Haines DD, Tosaki A, Bak I (2017) Antioxidant properties and oxidative transformation of different chromone derivatives. Molecules 22:588–599
DeLano WL (2002) PyMOL: an open-source molecular graphics tool. CCP4 Newsletter on Protein. Crystallography 40:82–92
Dennington R, Keith T, Millan J (2012) GaussView, Version 5. Semichem, Inc., Shawnee Mission, KS
Dofe VS, Sarkate AP, Lokwani DK, Shinde DB, Kathwate SH, Gill CH (2017a) Novel o-alkylated chromones as antimicrobial agents: ultrasound mediated synthesis, molecular docking and ADME prediction. J Heterocycl Chem 54:2678–2685
Dofe VS, Sarkate AP, Lokwani DK, Kathwate SH, Gill CH (2017b) Synthesis, antimicrobial evaluation, and molecular docking studies of novel chromone based 1,2,3-triazoles. Res Chem Intermed 43:15–28
Farrugia LJ (1997) ORTEP-3 for Windows—a version of ORTEP-III with a Graphical User Interface (GUI). J Appl Cryst 30:565
Farrugia LJ (1999) WinGX suite for small-molecule single-crystal crystallography. J Appl Cryst 32:837–838
Farrugia LJ (2012) WinGX and ORTEP for Windows: an update. J Appl Cryst 45:849–854
Gaspar A, Matos MJ, Garrido J, Uriarte E, Borges F (2014) Chromone: a valid scaffold in medicinal chemistry. Chem Rev 114:4960–4992
Goto H, Terao Y, Akai S (2009) Synthesis of various kinds of isoflavones, isoflavones, and biphenyl-ketones and their 1,1-diphenyl-2-picrylhydrazyl radical-scavenging activities. Chem Pharm Bull 57:346–360
Huls R (1958) Synthesis of substituted chromones. Synthesis of 6,7-dimethoxy-2,2-dimethyl-3-chromene (ageratochromone) Bull Soc Chim Belg 67:22–32
Jerezano A, Jiménez F, Cruz MC, Montiel LE, Delgado F, Tamariz J (2011) New approach for the construction of the coumarin frame and application in the total synthesis of natural products. Helv Chim Acta 94:185–198
Jha HC, Zilliken F, Offermann W, Breitmaier E (1981) Carbon-13 NMR shifts and C–H coupling constants of deoxybenzoins and related acetophenones. Can J Chem 59:2266–2281
Jiménez F, Cruz MC, Zúñiga C, Martínez MA, Chamorro G, Díaz F, Tamariz J (2010) Aryloxyacetic ester structurally related to α-asarone as potential antifungal agents. Med Chem Res 19:33–57
Jhon CH, Riyaphan J, Lin SH, Chia YC, Weng CF (2015) Screening alpha-glucosidase and alpha-amylase inhibitors from natural compounds by molecular docking in silico. Biofactors 41:242–251
Jose ES, Philip JE, Shanty AA, Kurup MRP, Mohanan PV (2018) Novel class of mononuclear 2-methoxy-4-chromanones ligated Cu (II), Zn (II), Ni (II) complexes: synthesis, characterization and biological studies. Inorg Chim Acta 478:155–165
Keri RS, Budagumpi S, Krishna R, Balakrishna RG (2014) Chromones as a privileged scaffold in drug discovery: a review. Eur J Med Chem 78:340–374
Kerru N, Singh-Pillay A, Awolade P, Singh P (2018) Current antidiabetic agents and their molecular targets: a review. Eur J Med Chem 152:436–488
Kim MK, Yoon H, Barnard DL, Chong Y (2013) Design, synthesis and antiviral activity of 2-(3-amino-4-piperazinylphenyl)chromone derivatives. Chem Pharm Bull 61:486–488
Labarrios E, Jerezano A, Jiménez F, Cruz MC, Delgado F, Zepeda JG, Tamariz J (2014) Efficient synthetic approach to substituted benzo[b]furans and benzo[b]thiophenenes by iodine-promoted cyclization of enaminones. J Heterocycl Chem 51:954–971
Lu M, Li T, Wan J, Li X, Yuan L, Sun S (2017) Antifungal effects of phytocompounds on Candida species alone and in combination with fluconazole. Int J Antimicrob Agents 49:125–136
Mendieta A, Jiménez F, Garduño-Siciliano L, Mojica-Villegas A, Rosales-Acosta B, Villa-Tanaca L, Chamorro-Cevallos G, Medina-Franco JL, Meurice N, Gutiérrez RU, Montiel LE, Cruz MC, Tamariz J (2014) Synthesis and highly potent hypolipidemic activity of alpha-asarone- and fibrate-based 2-acyl and 2-alkyl phenols as HMG-CoA reductase inhibitors. Bioorg Med Chem 22:5871–5882
Moore ML, Day AA, Suter CM (1934) The preparation and germicidal properties of some derivatives of 4-n-butylresorcinol. J Am Chem Soc 56:2456–2459
Morris GM, Huey R, Lindstrom W, Sanner MF, Belew RK, Goodsell DS, Olson AJ (2009) AutoDock4 and AutoDock Tools4: automated docking with selective receptor flexibility. J Comput Chem 30:2785–2791
Musso L, Dallavalle S, Merlini L, Farina G (2010) Synthesis and antifungal activity of 2-hydroxy-4,4-methylenedioxyaryl ketones as analogues of kakuol. Chem Biodivers 7:887–897
Nohara A, Umetani T, Sanno Y (1974) Studies on antianaphylactic agents. I. Facile synthesis of 4-oxo-4H-1-benzopyran-3-carbaldehydes by Vilsmeier reagents. Tetrahedron 30:3553–3561
Philip JE, Shahid M, Prathapachandra Kurup MR, Velayudhan MP (2017) Metal based biologically active compounds: design, synthesis, DNA binding and antidiabetic activity of 6-methyl-3-formyl chromone derived. J Photochem Photobiol B 175:178–191
Rao YJ, Krupadanam GLD (1994) Facile synthesis of linear and angular 2-methylfurobenzopyranone. Bull Chem Soc Jpn 67:1972–1975
Reis J, Gaspar A, Milhazes N, Borges F (2017) Chromones as a privileged scaffold in drug discovery: recent advances. J Med Chem 60:7941–7957
Salehi P, Asghari B, Esmaeili MA, Dehghan H, Ghazi I (2013) α-Glucosidase and α-amylase inhibitory effect and antioxidant of ten plant extracts traditionally used in Iran for diabetes. J Med Plants Res 7:257–266
Sarker SD, Nahar L, kumarasamy Y (2007) Microtitre plate-based antibacterial assay incorporating resazurin as an indicator of cell growth, and its application in the in vitro antibacterial screening of phytochemicals. Methods 42:321–324
Seleem D, Pardi V, Murata RM (2017) Review of flavonoids: a diverse group of natural compounds with anti-Candida albicans activity in vitro. Arch Oral Biol 76:76–83
Sheldrick GM (2008) A short history of SHELX. Acta Cryst Sect A 64:112–122
Sheldrick GM (2015) SHELXT—integrated space-group and crystal-structure determination. Acta Cryst Sect A 71:3–8
Soengas RG, Silva VLM, Ide D, Kato A, Cardoso SM, Almeida FAP, Silva AMS (2016) Synthesis of 3-(2-nitrovinyl)-4H-chromones: useful scaffolds for the construction of biological relevant 3-(pyrazol-5-yl)chromones. Tetrahedron 72:3198–3203
Sun YL, Bao J, Liu KS, Zhang XY, He F, Wang YF, Nong XH, Qi SH (2013) Cytotoxic dihydrothiophene-condensed chromones from the marine-derived fungus Penicillium oxacilum. Planta Med 79:1474–1479
Takao K, Ishikawa R, Sugita Y (2014) Synthesis and biological evaluation of 3-styrylchromones derivatives as free radical scavengers and α-glucosidase inhibitors. Chem Pharm Bull 62:810–815
Upadhyay J, Polyzos SA, Perakakis N, Thakkar B, Paschou SA, Katsiki N, Underwood P, Park KH, Seufert J, Kang ES, Sternthal E, Karagiannis A, Mantzoros CS (2018) Pharmacotherapy of type 2 diabetes: an update. Metabolism 78:13–42
Wang G, Chen M, Wang J, Peng Y, Li L, Xie Z, Deng B, Chen S, Li W (2017) Synthesis, biological evaluation and molecular docking studies of chromone hydrazine derivatives as α-glucosidase inhibitors. Bioorg Med Chem Lett 27:2957–2961
Wang G, Chen M, Qiu J, Xie Z, Cao A (2018) Synthesis, in vitro α-glucosidase inhibitory activity and docking studies of novel chromone-isatin derivatives. Bioorg Med Chem Lett 28:113–116
Whaley SG, Berkow EL, Rybak JM, Nishimoto AT, Barker KS, Rogers PD (2017) Azole antifungal resistance in Candida albicans and emerging non-albicans Candida species. Front Microbiol 7:2173–2184
Xiu C, Hua Z, Xiao BS, Tang WJ, Zhou HP, Liu XH (2017) Novel benzopyran derivatives and their therapeutic applications: a patent review (2009–2016). Expert Opin Ther Pat 27:1031–1045
Zhen J, Dai Y, Villani T, Giurleo D, Simon JE, Wu Q (2017) Synthesis of novel flavonoid alkaloids as α-glucosidase inhibitors. Bioorg Med Chem 25:5355–5364
Zou H, Jiang H, Zhou JY, Zhu Y, Cao L, Xia P, Zhang Q (2010) Synthesis of 3-(4-hydroxyphenyl)-4-methyl-6-methoxy-7-hydroxycoumarin and its analogous as angiogenesis inhibitors. J Chin Pharm Sci 19:136–140
Acknowledgements
The authors thank Carlos Espinoza-Hicks for help in spectrometric measurements and Bruce A. Larsen for proofreading. J.T. acknowledges financial support from the Secretaría de Investigación y Posgrado, Instituto Politécnico Nacional (SIP-IPN) (grants 20090326, 20100236, 20110172, 20120830, 20130686, 20140858, 20180198, and 20195228) and CONACYT (grants 83446 and 178319). For financial support, F.D. is grateful to SIP-IPN (grants 20170925, 20181332, and 20195287) and J.C.-B. to CONACyT (CBB-254600 and APN-782). A.M.-M. and C.R.-E. thank CONACYT for the graduate scholarships awarded (178319 and 264517) and SIP-IPN (BEIFI) for a complementary scholarship. This work was financially supported by a project from SIP-IPN (20170430, 20181433, 20195786). F.E.J.-M. (20160247), M.C.C.-L. (20160206), F.D., and J.T. are fellows of the Estímulos al Desempeño de los Investigadores (EDI-IPN), and M.C.C.-L., F.D., and J.T. are fellows of the Comisión de Operación y Fomento de Actividades Académicas (COFAA)-IPN.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Mendieta-Moctezuma, A., Rugerio-Escalona, C., Villa-Ruano, N. et al. Synthesis and biological evaluation of novel chromonyl enaminones as α-glucosidase inhibitors. Med Chem Res 28, 831–848 (2019). https://doi.org/10.1007/s00044-019-02320-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00044-019-02320-w