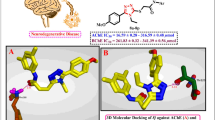
Abstract
In this study we report that amino acridine intermediates 7 and 8 were obtained from the reduction of nitro acridine derivatives 5 and 6 that were synthesized via the condensation of dimedone, p-nitrobenzaldehyde with various amine derivatives, respectively. Then acridine sulfonamide hybrid compounds (9–18) were synthesized by the reaction of amino acridine 7, 8 with sulfonyl chlorides. The new hybrid compounds were characterized by FT-IR, 1H-NMR, 13C-NMR, and HRMS analyzes. The evaluation of in vitro anticholinesterase action of the synthesized compounds against AChE showed that some of them are potent inhibitors. Among them, compound 17 showed the most potent activity against AChE with an IC50 of 0.14 µM.


Similar content being viewed by others
References
Anand P, Singh B (2013) A review on cholinesterase inhibitors for Alzheimer’s disease. Arch Pharmacal Res 36:375–399
Antonini I, Polucci P, Kelland RL, Menta E, Pescalli N, Martelli S (1999) 2,3-dihydro-1H,7H-pyrimido[5,6,1-de]acridine-1,3,7-trione derivatives, a class of cytotoxic agents active on multidrug-resistant cell lines: synthesis, biological evaluation, and structure−activity relationships. J Med Chem 42:2535–2541
Bag S, Tulsan R, Sood A, Cho H, Redjeb H, Zhou W, LeVine H, Török B, Török M (2015) Sulfonamides as multifunctional agents for Alzheimer’s disease. Bioorg Med Chem Lett 25:626–630
Bosak A, Gazić I, Vinković V, Kovarik Z (2008) Stereoselective inhibition of human, mouse, and horse cholinesterases by bambuterol enantiomers. Chem Biol Interact 175:192–195
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Ellman GL, Courtney KD, Andres V, Feather-Stone RM (1961) A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem Pharm 7:88–95
Gallo S, Atifi S, Mahamoud A, Santelli-Rouvier C, Wolfart K, Molnar J (2003) Synthesis of aza mono, bi and tricyclic compounds. Evaluation of their anti MDR activity. Eur J Med Chem 38:19–26
Hamulakovaa S, Imricha J, Janoveca L, Kristiana P, Danihela I, Holasb O, Pohankac M, Böhmd S, Kozurkovaa M, Kucac K (2014) Novel tacrine/acridine anticholinesterase inhibitors with piperazine and thiourea linkers. Int J Biol Macromol 70:435–439
Kaya M, Basar E, Çakir E, Tunca E, Bulbul M (2012) Synthesis and characterization of novel dioxoacridine sulfonamide derivatives as new carbonic anhydrase inhibitors. J Enzyme Inhib Med Chem 27:509–514
Kaya M, Demir E, Bekci H (2013) Synthesis, characterization and antimicrobial activity of novel xanthene sulfonamide and carboxamide derivatives. J Enzyme Inhib Med Chem 28:885–893
Kaya M, Yıldırır Y, Çelik GY (2011) Synthesis and antimicrobial activities of novel bisacridine-1,8-dione derivatives. Med Chem Res 20:293–299
Marco-Contelles J, León R, Ríos CL, Samadi A, Bartolini M, Andrisano V, Huertas O, Barril X, Luque FJ, Rodríguez-Franco MI, López B, López MG, García AG, Carreiras MC, Villarroya M (2009) Tacripyrines, the first tacrine−dihydropyridine hybrids, as multitarget-directed ligands for the treatment of alzheimer’s disease. J Med Chem 52:2724–2732
Monti SM, Supuran CT, Simone GD (2013) Anticancer carbonic anhydrase inhibitors: a patent review. Expert Opin Ther Patents 23:737–749
Öcal B, Saraç B, Simsek R, Yıldırım S, Sarıoğlu Y, Safak C (2002) Vasorelaxing properties of some phenylacridine type potassium channel openers in isolated rabbit thoracic arteries. Eur J Med Chem 37:519–523
Palani K, Thirumalai D, Ambalavanan P, Ponnuswamy MN, Ramakrishnan VT (2005) Synthesis and characterization of 9-(4-nitrophenyl)3,3,6,6-tetramethyl-3,4,6,7,9,10-hexahydro-1,8(2H,5H) acridinedione and its methoxyphenyl derivative. J Chem Crystallogr 35:751–760
Pamuk H, Aday B, Şen F, Kaya M (2015) Pt NPs@GO as a highly efficient and reusable catalyst for one-pot synthesis of acridinedione derivatives. RSC Adv 5:49295–49300
Scozzafava A, Supuran CT, Carta F (2013) Antiobesity carbonic anhydrase inhibitors: a literature and patent review. Expert Opin Ther Patents 23:725–735
Silman I, Sussman JL (2005) Acetylcholinesterase: ‘classical’ and ‘non-classical’ functions and pharmacology. Curr Opin Pharmacol 5:293–302
Slawinski J, Brzozowski Z, Zolnowska B, Szafranski K, Pogorzelska A, Vullo D, Supuran CT (2014a) Synthesis of a new series of N 4-substituted 4-(2-aminoethyl)benzenesulfonamides and their inhibitory effect on human carbonic anhydrase cytosolic isozymes I and II and transmembrane tumor-associated isozymes IX and XII. Eur J Med Chem 84:59–67
Slawinski J, Pogorzelska A, Zolnowska B, Brozewicz K, Vullo D, Supuran CT (2014b) Carbonic anhydrase inhibitors. Synthesis of a novel series of 5-substituted 2,4-dichlorobenzenesulfonamides and their inhibition of human cytosolic isozymes I and II and the transmembrane tumor-associated isozymes IX and XII. Eur J Med Chem 82:47–55
Soreq H, Seidman S (2001) Acetylcholinesterase-new roles for an old actor. Nat Rev Neurosci 2:294–302
Srivastava A, Nizamuddin C (2004) Synthesis and fungicidal activity of some acridine derivatives. Indian J Heterocycl Chem 13:261–264
Supuran CT, Scozzafava A (2001) Carbonic anhydrase inhibitors. Curr Med Chem 1:61–97
Talesa V, Principato E, Di Giovannini MV, Grauso G, Rosi G (1993) Evidence for a molecular polimorphism of cholinesterase in sepia officinalis. Comp Biochem Physiol B 106:557–562
Tang ZQ, Chen Y, Liu CN, Cai KY, Tuc SJ (2010) A green procedure for the synthesis of 1,8-dioxodecahydroacridine derivatives under microwave irradiation in aqueous media without catalyst. J Heterocyclic Chem 47:363–367
Thullbery MD, Cox HD, Schule T, Thompson CM, George KM (2005) Differential localization of acetylcholinesterase in neuronal and non-neuronal cells. J Cell Biochem 96:599–610
Ulus R, Yeşildağ İ, Elmastaş M, Kaya M (2015) Rapid synthesis of novel 1,8-dioxoacridine carboxylic acid derivatives by microwave irradiation and their free radical scavenging activity. Med Chem Res 24:3752–3759
Ulus R, Yeşildağ İ, Tanc M, Bülbül M, Kaya M, Supuran CT (2013) Synthesis of novel acridine and bis acridine sulfonamides with effective inhibitory activity against the cytosolic carbonic anhydrase isoforms II and VII. Bioorg Med Chem 21:5799–5805
Yeşildağ İ, Ulus R, Basar E, Aslan M, Kaya M, Bülbül M (2014) Facile, highly efficient, and clean one-pot synthesis of acridine sulfonamide derivatives at room temperature and their inhibition of human carbonic anhydrase isoenzymes. Monatsh Chem 145:1027–1034
Zolnowska B, Slawinski J, Pogorzelska A, Chojnacki J, Vullo D, Supuran CT (2014) Carbonic anhydrase inhibitors. Synthesis, and molecular structure of novel series N-substituted N′-(2-arylmethylthio-4-chloro-5-methylbenzenesulfonyl) guanidines and their inhibition of human cytosolic isozymes I and II and the transmembrane tumor-associated isozymes IX and XII. Eur J Med Chem 71:135–147
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Ulus, R., Esirden, İ., Aday, B. et al. Synthesis of novel acridine-sulfonamide hybrid compounds as acetylcholinesterase inhibitor for the treatment of alzheimer’s disease. Med Chem Res 27, 634–641 (2018). https://doi.org/10.1007/s00044-017-2088-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00044-017-2088-2