Abstract
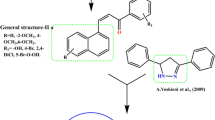
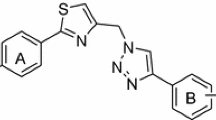
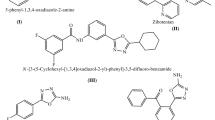
Herein, we describe the synthesis of 11new thiazolyl coumarin derivatives and evaluation of their potential role as antibacterial and antituberculosis agents. The structures of the synthesized compounds were established by extensive spectroscopic studies (Fourier transform infrared spectroscopy, 1H-nuclear magnetic resonance, 13C-nuclear magnetic resonance, 2D-nuclear magnetic resonance and liquid chromatography–mass spectrometry) and elemental analysis. All synthesized compounds were assayed for their in vitro antibacterial activity against a few gram positive and gram negative bacteria and antituberculosis activity against Mycobacterium tuberculosis H37Rv ATCC 25618 by using colorimetric microdilution assay method. Nine derivatives showed moderate anti-bacterial and anti-tuberculosis activities against all the tested strains. The highest activity against all the pathogens including Mycobacterium tuberculosis was observed by compound 7c with MIC values ranging between 31.25–62.5 μg/mL, indicating that coumarin skeleton could indeed provide useful scaffold for the development of new anti-microbial drugs.




Similar content being viewed by others
References
Abdelgadir HA, Van Staden J (2013) Ethnobotany, ethnopharmacology and toxicity of Jatropha curcas L. (Euphorbiaceae): A review. S Afr J Bot 88:204–218
Arshad A, Osman H, Bagley MC, Lam CK, Mohamad S, Mohdzahariluddin AS (2011) Synthesis and antimicrobial properties of some new thiazolyl coumarin derivatives. Eur J Med Chem 46:3788–3794
Arya V (2011) A review on anti-tubercular plants. Int J Phar Tech Res 3:872–80
Atre S (2015) An urgent need for building technical capacity for rapid diagnosis of multidrug-resistant tuberculosis (MDR-TB) among new cases: A case report from Maharashtra, India. J Infect Public Health 8(5):502–505
Azelmat J, Fiorito S, Taddeo VA, Genovese S, Epifano F, Grenier D (2015) Synthesis and evaluation of antibacterial and anti-inflammatory properties of naturally occurring coumarins. Phytochem Lett 13:399–405
Bjarnsholt T (2013) The role of bacterial biofilms in chronic infections. Acta Pathol Microbiol Immunol Scand 121(s136):1–58
Deribew A, Deribe K, Reda AA, Tesfaye M, Hailmichael Y, Maja T (2013) Do common mental disorders decline over time in TB/HIV co-infected and HIV patients without TB who are on antiretroviral treatment? BMC psychiatry 13(174):1–6
Elhalim SA, Ibrahim IT (2015) Radioiodination of 2, 3-dimethyl-4H-furo [3, 2-c] coumarin and biological evaluation in solid tumor bearing mice. Appl Radiat Isot 95:153–158
Elmi OS, Jeab MZ, Abdullah SB, Hasan H, Zilafil BA, Naing NN (2016) Extensively drug-resistant mycobacterium tuberculosis in Kelantan east coast of Malaysia: first two cases. Clin Microbiol Newsl 38(5):40–42
Ghosh S, Indukuri K, Bondalapati S, Saikia AK, Rangan L (2013) Unveiling the mode of action of antibacterial labdane diterpenes from Alpinia nigra (Gaertn.) B. L. Burtt seeds. Eur J Med Chem 66:101–105
Gursoy A, Karali N (2003) Synthesis, characterization and primary antituberculosis activity evaluation of 4-(3-coumarinyl)-3-benzyl-4-thiazolin-2-one benzylidenehydrazones. Turk J Chem 27(5):545–52
Hasanen JA (2012) Synthesis and mass spectra of some new 3-substituted coumarin derivatives. Der Pharma Chem 4:1923–1934
Hummelova J, Rondevaldova J, Balastikova A, Lapcik O, Kokoska L (2014) The relationship between structure and in vitro antibacterial activity of selected isoflavones and their metabolites with special focus on antistaphylococcal effect of demethyltexasin. Lett Appl Microbiol 60:242–247
Joshi JM (2011) Tuberculosis chemotherapy in the 21st century: Back to the basics. Lung India 28(3):193–200
Karatas MO, Olgundeniz B, Gunal S, Ozdemir I, Alici B, Cetinkaya E (2015) Synthesis, characterization and antimicrobial activities of novel silver (I) complexes with coumarin substituted N-heterocyclic carbene ligands. Bioorg Med Chem 24(4):643–650
Kharb R, Shahar Yar M, Sharma PC (2011) New insights into chemistry and anti-infective potential of triazole scaffold. Curr Med Chem 18(21):3265–3297
Kumar HN, Parumasivam T, Jumaat F, Ibrahim P, Asmawi MZ, Sadikun A (2014) Synthesis and evaluation of isonicotinoyl hydrazone derivatives as antimycobacterial and anticancer agents. Med Chem Res 23(1):269–279
Ma C, Case RJ, Wang Y, Zhang HJ, Tan GT, Van Hung N, Cuong NM, Franzblau SG, Soejarto DD, Fong HH, Pauli GF (2005) Anti-tuberculosis constituents from the stem bark of Micromelum hirsutum. Planta Med 71(3):261–267
Mahato S, Singh A, Rangan L, Jana CK (2016) Synthesis, In silico studies and In vitro evaluation for antioxidant and antibacterial properties of diarylmethylamines: A novel class of structurally simple and highly potent pharmacophore. Eur J of Pharm Sci 88:202–209
Mannhold R, Kubinyi H, Timmerman H (2008) Lipophilicity in drug action and toxicology (Vol 4), Pliska V, Testa B, van de Waterbeemd H (eds). Wiley-VCH Verlag GmbH: 26 Sep
Matsuda K, Hattori S, Kariya R, Komizu Y, Kudo E, Goto H, Taura M, Ueoka R, Kimura S, Okada S (2015) Inhibition of HIV-1 entry by the tricyclic coumarin GUT-70 through the modification of membrane fluidity. Biochem Biophys Res Commun 457(3):288–294
Muthukrishnan M, Mujahid M, Yogeeswari P, Sriram D (2011) Syntheses and biological evaluation of new triazole-spirochromone conjugates as inhibitors of Mycobacterium tuberculosis. Tetrahedron Lett 52(18):2387–2389
Nocentini A, Carta F, Ceruso M, Bartolucci G, Supuran CT (2015) Click-tailed coumarins with potent and selective inhibitory action against the tumor-associated carbonic anhydrases IX and XII. Bioorg Med Chem 23(21):6955–6966
Patil SA, Prabhakara CT, Halasangi BM, Toragalmath SS, Badami PS (2015) DNA cleavage, antibacterial, antifungal and anthelmintic studies of Co (II), Ni (II) and Cu (II) complexes of coumarin Schiff bases: Synthesis and spectral approach. Spectrochim Acta A Mol Biomol Spectrosc 137:641–651
Prakash R, Kumar D, Gupta VK, Jain S, Chauhan DS, Tiwari PK, Katoch VM (2016) Status of multidrug resistant tuberculosis (MDR-TB) among the Sahariya tribe of North Central India. J Infect Public Health 9(3):289–297
Prasad R, Srivastava DK (2013) Multi drug and extensively drug-resistant TB (M/XDR-TB) management: Current issues. Clin Epidemiol Glob Health 1(3):124–128
Shaikh MH, Subhedar DD, Khan FA, Sangshetti JN, Shingate BB (2016) 1, 2, 3-Triazole incorporated coumarin derivatives as potential antifungal and antioxidant agents. Chin Chem Lett 2(27):295–301
Vazquez-Rodriguez S, Lopez RL, Matos MJ, Armesto-Quintas G, Serra S, Uriarte E, Santana L, Borges F, Crego AM, Santos Y (2015) Design, synthesis and antibacterial study of new potent and selective coumarin–chalcone derivatives for the treatment of tenacibaculosis. Bioorg Med Chem 23(21):7045–7052
World Health Organisation Global Tuberculosis report 2015. http://www.who.int/tb/publications/global_report/gtbr15_main_text.pdf. Accessed 10 June 2016. WHO/ HTM/TB/2015
Acknowledgements
The authors thank the School of Chemical Sciences, Universiti Sains Malaysia (USM) for providing necessary research facilities. The authors are also thankful to the School of Biological Sciences, USM and Faculty of Science and Natural Resources, University Malaysia Sabah for providing facilities for biological studies. Thanks are due to Malaysian Government and USM for the grant FRGS 203/PKIMIA/6711462 to conduct this work. Samina Khan Yusufzai thanks Graduate Assistance fellowship for financial support.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interest.
Electronic supplementary material
Rights and permissions
About this article
Cite this article
KhanYusufzai, S., Osman, H., Khan, M.S. et al. Design, characterization, in vitro antibacterial, antitubercular evaluation and structure–activity relationships of new hydrazinyl thiazolyl coumarin derivatives. Med Chem Res 26, 1139–1148 (2017). https://doi.org/10.1007/s00044-017-1820-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00044-017-1820-2