Abstract
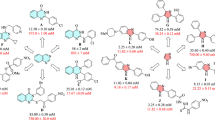
In this paper, we report a series of eighteen novel pyrimidine-based thiourea compounds with good to excellent yields (61–88%). The chemical structures of these heterocycles consist of a central pyrimidine ring with phenyl-substituted thiourea motifs. The enzyme inhibitory potential of these compounds was investigated against α-glucosidase as this enzyme plays a crucial role in treating type II diabetes mellitus. Compounds 4i (IC50 = 22.46 ± 0.65 µM), 4f (IC50 = 25.88 ± 0.40 µM), 4h (IC50 = 27.63 ± 0.49 µM), 4c (IC50 = 29.47 ± 0.42 µM), and 4e (IC50 = 32.01 ± 0.42 µM) delivered better inhibition than the reference compound acarbose (IC50 38.22 ± 0.12 µM). The quantitative structure–activity relationship of the synthesized compounds was also studied.



Similar content being viewed by others
References
Alagarsamy V, Salomon VR, Vanikavitha G, Paluchamy V, Chandran MR, Sujin AA, Thangathiruppathy A, Amuthalakshmi S, Revathi R (2002) Synthesis, analgesic, anti-inflammatory and antibacterial activities of some novel 2-phenyl-3-substituted quinazolin-4 (3H) ones. Biol Pharm Bull 25(11):1432–1435
Bahekar SS, Shinde DB (2004) Synthesis and anti-inflammatory activity of some [4, 6- (4-substituted aryl)-2-thioxo-1, 2, 3, 4-tetrahydro-pyrimidin-5-yl]-acetic acid derivatives. Bioorg Med Chem Lett 14(7):1733–1736
Bernier J, Henichart J, Warin V, Baert F (1980) Synthesis and structure–activity relationship of a pyrimido [4, 5‐d] pyrimidine derivative with antidepressant activity. J Pharm Sci 69(11):1343–1345
Chapdelaine P, Tremblay RR, Dube J (1978) P-nitrophenol-alpha-d-glucopyranoside as substrate for measurement of maltase activity in human semen. Clin Chem 24(2):208–211
Ermert P, Vasella A (1993) A new approach to 5‐thiosugars: 5‐thio‐d‐gluconhydroximo‐1,5‐lactone, synthesis and evaluation as β‐glucosidase inhibitor.. Helv Chim Acta 76(7):2687–2699
Ferreira SB, Sodero AC, Cardoso MF, Lima ES, Kaiser CR, Silva Jr FP, Ferreira VF (2010) Synthesis, biological activity, and molecular modeling studies of 1 H-1, 2, 3-triazole derivatives of carbohydrates as α-glucosidases inhibitors. J Med Chem 53(6):2364–2375
Gadhachanda VR, Wu B, Wang Z, Kuhen KL, Caldwell J, Zondler H, Walter H, Havenhand M, He Y (2007) 4-Aminopyrimidines as novel HIV-1 inhibitors. Bioorg Med Chem Lett 17(1):260–265
García-Moreno MI, Rodríguez-Lucena D, Mellet CO, García Fernández JM (2004) Pseudoamide-type pyrrolidine and pyrrolizidine glycomimetics and their inhibitory activities against glycosidases. J Org Chem 69(10):3578–3581
Gong B, Hong F, Kohm C, Jenkins S, Tulinsky J, Bhatt R, de Vries P, Singer JW, Klein P (2004) Synthesis, SAR, and antitumor properties of diamino-C, N-diarylpyrimidine positional isomers: inhibitors of lysophosphatidic acid acyltransferase-β. Bioorg Med Chem Lett 14(9):2303–2308
Hawser S, Lociuro S, Islam K (2006) Dihydrofolate reductase inhibitors as antibacterial agents. Biochem Pharmacol 71(7):941–948
Hocková D, Holý A, Masojídková M, Andrei G, Snoeck R, De Clercq E, Balzarini J (2003) 5-Substituted-2, 4-diamino-6-[2-(phosphonomethoxy) ethoxy] pyrimidines acyclic nucleoside phosphonate analogues with antiviral activity. J Med Chem 46(23):5064–5073
Holla BS, Malini K, Rao BS, Sarojini B, Kumari NS (2003) Synthesis of some new 2, 4-disubstituted thiazoles as possible antibacterial and anti-inflammatory agents. Eur J Med Chem 38(3):313–318
Imran S, Taha M, Ismail NH, Fayyaz S, Khan KM, Choudhary MI (2015) Synthesis, biological evaluation, and docking studies of novel thiourea derivatives of bisindolylmethane as carbonic anhydrase II inhibitor. Bioorg Chem 62:83–93
Jeong LS, Zhao LX, Choi WJ, Pal S, Park YH, Lee SK, Chun MW, Lee YB, Ahn CH, Moon HR (2007) Synthesis and antitumor activity of fluorocyclopentenyl-pyrimidines. Nucleosides Nucleotides Nucleic Acids 26(6–7):713–716
Keche AP, Hatnapure GD, Tale RH, Rodge AH, Birajdar SS, Kamble VM (2012) A novel pyrimidine derivatives with aryl urea, thiourea and sulfonamide moieties: synthesis, anti-inflammatory and antimicrobial evaluation. Bioorg Med Chem Lett 22(10):3445–3448
Lee J, Kim K-H, Jeong S (2011) Discovery of a novel class of 2-aminopyrimidines as CDK1 and CDK2 inhibitors. Bioorg Med Chem Lett 21(14):4203–4205
Milling RJ, Richardson CJ (1995) Mode of action of the anilino‐pyrimidine fungicide pyrimethanil. 2. Effects on enzyme secretion in Botrytis cinerea. Pestic Sci 45(1):43–48
Nie A, Wang J, Huang Z (2006) Microwave-assisted solution-phase parallel synthesis of 2, 4, 6-trisubstituted pyrimidines. J Comb Chem 8(5):646–648
Riaz S, Khan IU, Bajda M, Ashraf M, Shaukat A, Rehman TU, Mutahir S, Hussain S, Mustafa G, Yar M (2015) Pyridine sulfonamide as a small key organic molecule for the potential treatment of type-II diabetes mellitus and alzheimer’s disease: in vitro studies against yeast α-glucosidase, acetylcholinesterase and butyrylcholinesterase. Bioorg Chem 63:64–71
Riaz S, Khan IU, Yar M, Ashraf M, Rehman TU, Shaukat A, Jamal SB, Duarte VC, Alves MJ (2014) Novel pyridine-2, 4, 6-tricarbohydrazide derivatives: design, synthesis, characterization and in vitro biological evaluation as α-and β-glucosidase inhibitors. Bioorg Chem 57:148–154
Taha M, Ismail NH, Imran S, Rokei MQB, Saad SM, Khan KM (2015) Synthesis of new oxadiazole derivatives as α-glucosidase inhibitors. Bioorg Med Chem 23(15):4155–4162
Yar M, Bajda M, Mehmood RA, Sidra LR, Ullah N, Shahzadi L, Ashraf M, Ismail T, Shahzad SA, Khan ZA (2014) Design and synthesis of new dual binding site cholinesterase inhibitors: in vitro inhibition studies with in silico docking. Lett Drug Des Discov 11(3):331
Yar M, Bajda M, Shahzad S, Ullah N, Gilani MA, Ashraf M, Rauf A, Shaukat A (2015) Organocatalyzed solvent free an efficient novel synthesis of 2, 4, 5-trisubstituted imidazoles for α-glucosidase inhibition to treat diabetes. Bioorg Chem 58:65–71
Yar M, Sidra LR, Pontiki E, Mushtaq N, Ashraf M, Nasar R, Khan IU, Mahmood N, Naqvi SAR, Khan ZA (2014) Synthesis, in vitro lipoxygenase inhibition, docking study and thermal stability analyses of novel indole derivatives. J Iran Chem Soc 11(2):369–378
Zhao F, Xiao JH, Wang Y, Li S (2009) Synthesis of thiourea derivatives as CCR4 antagonists. Chin Chem Lett 20(3):296–299
Acknowledgements
We acknowledge Higher Education Commission of Pakistan for financial support under National Research Program for Universities (Project No. 3971).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The author declares that he has no competing interests.
Rights and permissions
About this article
Cite this article
Ur Rehman, T., Ullah Khan, I. & Riaz, S. Novel substituted 3-phenyl 1-(4-(5-bromopyridin-3-yl)-6-phenylpyrimidin-2-yl)-thiourea compounds as key small organic molecules for the potential treatment of type II diabetes mellitus: in vitro studies against yeast α-glucosidase. Med Chem Res 26, 1098–1106 (2017). https://doi.org/10.1007/s00044-017-1803-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00044-017-1803-3