Abstract
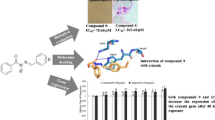
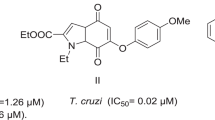
Chagas disease is a very important neglected disease and millions of people live in endemic areas at the risk of acquiring the infection. Due to scarce and ineffective current chemotherapy, the introduction of new drugs on therapeutics is highly necessary. Bioisoster hybrids derivatives were previously designed, synthesized, and assayed in terms of trypanocidal activity and permeability. Structure activity-relationships were performed with a set of N-acylhydrazone and furoxan derivatives aiming at identifying if the enzyme cruzain might be the target of the system. In addition, lowest unoccupied molecular orbital analysis and docking studies of the two most promising compounds were carried out with the purpose of elucidating the key properties and interactions between the ligands and cruzain. The analysis of cruzain inhibition tests showed that the position of the nitro group is important for the compounds inhibitory activity. The results lead to the conclusion that cruzain may not be the only target. This hypothesis was advanced because the most active in Trypanosoma cruzi, compound 6, is not the most effective in cruzain tests. Notwithstanding, this compound was considered the most promising hit for further in vivo studies, as it did not show toxicity in cycle cell tests.






Similar content being viewed by others
References
Allen FH (2002) The Cambridge structural database: a quarter of a million crystal structures and rising. Acta Crystallogr B 58:380–388
Anis RJ, Anis R, Marin-Neto JA (2010) Chagas disease. Lancet 375:1388–1402
Borchardt DM, Mascarello A, Chiaradia LD, Nunes RJ, Oliva G, Yunes RA, Andricopulo AD (2010) Biochemical evaluation of a series of synthetic chalcone and hydrazide derivatives as novel inhibitors of cruzain from Trypanosoma cruzi. J Braz Chem Soc 21:142–150
Coura JR, Dias JCP (2009) Epidemiology, control and surveillance of Chagas disease – 100 years after its discovery. Mem Inst Oswaldo Cruz 104:31–40
Dias LC, Dessoy MA, Silva JNS, Thiemann OH, Oliva G, Andricopulo AD (2009) Quimioterapia da doença de Chagas: estado da arte e perspectivas no desenvolvimento de novos fármacos. Quim Nova 32:2444–2457
DNDi, Drug for Neglected Disease Initiative (2016a) Chagas disease. http://www.dndi.org/diseases-projects/diseases/chagas.html. Accessed 5 Apr 2016
DNDi, Drug for Neglected Disease Initiative (2016b) Chagas disease: current treatment. http://www.dndi.org/diseases-projects/diseases/chagas/current-treatment.html. Accessed 5 April 2016
Doherty D (1997) MOLSIM: molecular mechanics and dynamics simulation software-user’s guide. The Chem21 group Inc, Lake Forest, Version 3.2
Eakin AE, Mills AA, Harth G, McKerrow JH, Craik CS (1992) The sequence, organization, and expression of the major cysteine protease (cruzain) from Trypanosoma cruzi. J Biol Chem 267:7411–7420
Gaussian 03W-revision B.02 for Windows (2003) Version 6, Gaussian Inc., Pittsburgh, PA
Gerets HHJ, Hanon E, Cornet M, Dhalluin S, Depelchin O, Canning M, Atienzar FA (2009) Selection of cytotoxicity markers for the screening of new chemical entities in a pharmaceutical context: a preliminary study using a multiplexing approach. Toxicol in Vitro 23:319–332
Giarolla J, Ferreira EI (2015) Drug design for neglected disease in Brazil. Mini Rev Med Chem 15:220–242
Hyperchem Program Release 7 for Windows (2002) Hypercube, Inc., Gainesville, FL
Judice WAS, Cezari MHS, Lima APCA, Scharfstein J, Chagas JR, Tersariol IL, Juliano MA, Juliano L (2001) Comparision of the specificity, stability and individual rate constants with respective activation parameters for the peptidase activity of cruzipain and its recombinant form, cruzain, from Trypanosoma cruzi. Eur J Biochem 268:6578–6586
Li R, Kenyon GL, Cohen FE, Chen X, Gong B, Dominguez JN, Davidson E, Kurzban G, Miller RE, Nuzum EO, Rosenthal PJ, McKerrow JH (1995) In vitro antimalarial activity of chalcones and their derivatives. J Med Chem 38:5031–5037
McGrath ME, Eakin AE, Engel JC, McKerrow JH, Fletterick RJ (1995) The crystal structure of cruzain: a therapeutic target for Chagas‘ disease. J Mol Biol 247:251–259
Moreira DRM, Leite ACL, Santos RR, Soares MB (2009) Approaches for the development of new anti-Trypanosoma cruzi agents. Curr Drugs Targets 10:212–231
Morris GM, Huey R, Lindstrom W, Sanner MF, Belew RK, Goodsell DS, Olson AJ (2009) Autodock4 and autodocktools4: automated docking with selective receptor flexibility. J Comp Chem 16:2785–2791
Mott BT, Ferreira RS, Simeonov A, Jadhav A, Ang KK, Leister W, Shen M, Silveira JT, Doyle PS, Arkin MR, McKerrow JH, Inglese J, Austin CP, Thomas CJ, Shoichet BK, Maloney DJ (2010) Indentification and optimization of inhibitors of Trypanosomal cysteine protease: cruzain, rhodesain, and TbCatB. J Med Chem 53:52–60
Nunes MCP, Dones W, Morillo CA, Encina JJ, Ribeiro AL (2013) Chagas disease an overview of clinical and epidemiological aspects. J Am Coll Cardiol 62:767–776
Requena-Mendez A, Aldasoro E, Lazzarari E, Sicuri E, Brown M, Moore DA, Gascon J, Muños J (2015) Prevalence of Chagas disease in latin-american migrants living in europe: a systematic review and meta-analysis. Plos Negl Trop Dis 9:1–15
Romeiro NC, Aguirre G, Hernandez P, González M, Cerecetto H, Aldana I, Pérez-Silanes S, Monge A, Barreiro EJ, Lima LM (2009) Synthesis, trypanocidal activity and docking studies of novel quinaxoline-N-acylhydrazones, designed as cruzain inhibitors candidates. Bioorg Med Chem 17:641–652
Sarsour EH, Kumar MG, Chaudhuri L, Kalen AL, Goswami PC (2009) Redox control of the cell cycle in health and disease. Antioxid Redox Signal 11:2985–3011
Schmunis GA, Yadon ZE (2010) Chagas disease: a latin American health problem becoming a world health problem. Acta Trop 115:14–21
Serafim RAM, Gonçalves JE, de Souza FP, Loureiro APM, Storpirtis S, Krogh R, Andricopulo AD, Dias LC, Ferreira EI (2014) Design, synthesis and biological evaluation of hybrid bioisoster derivatives of N-acylhydrazone and furoxan groups with potential and selective anti-Trypanosoma cruzi activity. Eur J Med Chem 82:418–425
Stewart JJP (2004) Optimization of parameters for semiempirical methods I. method. J Comput Chem 10:209–220
Taft CA, Silva VB, Silva CH (2008) Current topics in computer aided drug design. J Pharm Sci 97:1089–1098
Yoshiro R, Yasuo N, Inaoka DK, Hagiwara Y, Ohno K, Orita M, Inoue M, Shiba T, Harada S, Honma T, Balogun EO, da Rocha JR, Montanari CA, Kita K, Sekijima M (2015) Pharmacophore modeling for anti-chagas drug design using the fragment molecular orbital method. Plos One 10:1–15
WHO, World Health Organization (2016) Chagas disease. http://www.who.int/mediacentre/factsheets/fs340/en/. Accessed 5 April 2016
Acknowledgments
The authors thank Kerly Fernanda Mesquita Pasqualoto, PhD, from Instituto Butantan, Sao Paulo, for allowing the use of MOLSIM 3.2 software, which is under her responsibility, as well as Prof. Sandro Rogério de Almeida and Renata Chaves Albuquerque, from Faculdade de Ciências Farmacêuticas, USP, São Paulo, Brazil, for flow cytometer availability. They also want to thank CAPES for R.A.M. Serafim and T. F. Oliveira scholarships, CNPq for E. I. Ferreira, L. C. Dias, A. D. Andricopulo and A. P. M. Loureiro fellowships, and FAPESP, CNPq, and GlaxoSmithKline, for financial support.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Serafim, R.A.M., de Oliveira, T.F., Loureiro, A.P.M. et al. Molecular modeling and structure–activity relationships studies of bioisoster hybrids of N-acylhydrazone and furoxan groups on cruzain. Med Chem Res 26, 760–769 (2017). https://doi.org/10.1007/s00044-016-1776-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00044-016-1776-7