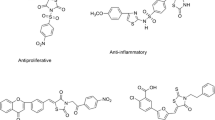
Abstract
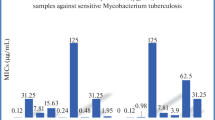
In order to explore various pharmacological effects associated with S-triazine accommodated 5-benzylidino-4-thiazolidinones, a series of compounds based on 5-benzylidene-2-[4-(4,6-bis-dimethylamino-[1,3,5]triazin-2-ylamino)-phenylimino]-thiazolidin-4-one were synthesized. Variation in the functional group at 5-benzylidine ring of thiazolidinone moiety led to a set of compounds bearing S-triazine accommodated with 4-thiazolidinones. Structures of the synthesized final compounds were confirmed by IR, 1H NMR, 13C NMR spectroscopy, ESI mass spectrometry, and elemental analysis. Synthesized compounds were screened against some bacterial and fungal strains using Kirby-Bauer disk diffusion technique and serial broth dilution technique. Synthesized compounds were screened to find out antitubercular activity against Mycobacterium tuberculosis H37RV by L. J. MIC method and BACTEC MGIT method. Relations between structure and its biological activities have been discussed.






Similar content being viewed by others
References
Anargyros P, Astill D, Lim I (1990) Comparison of improved BACTEC and Lowenstein-Jensen media for culture of mycobacteria from clinical specimens. J Clin Microbiol 28:1288–1291
Andres C, Bronson J, Andrea S, Deshpande M, Falk P, Grant-Young K, Harte W, Ho H, Misco P, Robertson J, Stock D, Sun Y, Walsh A (2000) 4-Thiazolidinones: novel inhibitors of the bacterial enzyme MurB. Bioorg Med Chem Lett 10:715–717
Arya K, Dandia A (2007) Synthesis and cytotoxicity of trisubstituted 1,3,5-triazines. Bioorg Med Chem Lett 17:3298–3304
Beemer P, Bodmer T, Munzinger J, Perrin M, Vincent V, Drugeon H (2004) Multicenter evaluation of fully automated BACTEC Mycobacteria Growth Indicator Tube 960 system for susceptibility testing of Mycobacterium tuberculosis. Clin Microbiol 42:1030–1034
Bruno G, Costantino L, Curinga C, Maccari R, Monforte F, Nicolo F, Ottana R, Vigorita M (2002) Synthesis and aldose reductase inhibitory activity of 5-arylidene-2,4-thiazolidinediones. Bioorg Med Chem 10:1077–1084
Carter P, Scherle P, Muckelbauer J, Voss M, Liu R, Thompson L, Tebben A, Solomon K, Lo Y, Li Z, Strzemienski P, Yang G, Falahatpisheh N, Xu M, Wu Z, Farrow N, Ramnarayan K, Wang J, Rideout D, Yalamoori V, Domaille P, Underwood D, Trzaskos J, Friedman S, Newton R, Decicco C (2001) Photochemically enhanced binding of small molecules to the tumor necrosis factor receptor-1 inhibits the binding of TNF-alpha. Proc Natl Acad Sci 98:11879–11884
Charlier C, Mishaux C (2003) Dual inhibition of cyclooxygenase-2(COX-2) and 5-lipoxygenase (5-LOX) as a new strategy to provide safer non-steroidal anti-inflammatory drugs. Eur J Med Chem 38:645–659
Collins C, Lyne P (1970) Antimicrobial susceptibility tests, chap 12. In: Collins and Lyne’s microbiological methods, 8th edn. Arnold, London, pp 168–185
Cutshall N, O’Day C, Prezhdo M (2005) Rhodanine derivatives as inhibitors of JSP-1. Bioorg Med Chem Lett 14:3374–3379
Desai A, Desai K (2005) Niementowski reaction: microwave induced and conventional synthesis of quinazolinones and 3-methyl- methyl-1H-5-pyrazolones and their antimicrobial activity. Arkivok 13:98–108
Desai K, Desai K (2006) Microbial screening of novel synthesized formazanes having amide linkages. J Heterocycl Chem 43:1083–1089
FDA Report-2012, <http://www.fda.gov/cder/approval/index.htm>
Gavade S, Markad V, Kodam K, Shingare M, Mane D (2012) Synthesis and biological evaluation of novel 2,4,6-triazine derivatives as antimicrobial agents. Bioorg Med Chem Lett 22:5075–5077
Global Tuberculosis Report-2012, WHO <http://www.who.int/tb/publications/global_report/en/>
Gududuru V, Hurh E, Dalton J, Miller D (2004) Synthesis and antiproliferative activity of 2-aryl-4-oxo-thiazolidin-3-yl-amides for prostate cancer. Bioorg Med Chem Lett 14:5289–5293
Hutchinson D (2004) Recent advances in oxazolidinone antibacterial agent research. Expert Opin Ther Pat 14:1309–1328
Kumar A, Srivastava K, Kumar S, Puri S, Chauhan P (2008) Synthesis and bioevaluation of hybrid 4-aminoquinoline triazines as a new class of antimalarial agents. Bioorg Med Chem Lett 18:6530–6533
Kumar A, Srivastava K, Kumar S, Puri S, Chauhan P (2009) Synthesis of 9-anilinoacridine triazine as new class of hybrid antimalarial agents. Bioorg Med Chem Lett 19:6996–6999
Mullen M, Daniel W, Wollum A (1988) Appl Environ Microbiol 54:2387–2392
Murray P, Baron E, Pfaller M, Tenevor F, Yolke R (1999) Manual for clinical microbiology, 7th edn. American Society for Microbiology
Patel N, Khan I (2011) Synthesis of 1,2,4-triazole derivatives containing benzothiazoles as pharmacologically active molecule. J Enzym Inhib Med Chem 26:527–534
Patel J, Mistry B, Desai K (2006) Synthesis and antimicrobial activity of newer quinazolinone. Eur J Chem 3:97–102
Rana A, Desai K, Jauhari S (2012) Synthesis and characterization of novel 2-[(5Z)-5-benzylidene-4-oxo-2-thioxo-1,3-thiazolidin-3-yl]-N-(2-methylphenyl)acetamide based analogues. Der Pharma Chemica 4(6):2453–2459
Rana A, Desai K, Jauhari S (2013) Rhodanine-based biologically active molecules: synthesis, characterization, and biological evaluation. Res Chem Intermed 2013. doi:10.1007/s11164-012-1001-3
Rana A, Desai K, Jauhari S (2013b) Synthesis, characterization, and pharmacological evaluation of 1-[2-(6-nitro-4-oxo-2-phenyl-4H-quinazolin-3-yl)-ethyl]-3-phenyl ureas. Med Chem Res 22:225–233
Saleh M, Abbott S, Perron V, Lauzon C, Penney C, Zacharie B (2010) Synthesis and antimicrobial activity of 2-fluorophenyl-4,6-disubstituted [1,3,5] triazines. Bioorg Med Chem Lett 20:945–949
Sim M, Ng S, Buss A, Crasta S, Goh K, Lee S (2002) Benzylidene rhodanines as novel inhibitors of UDP-nacetylmuramate/l-alanine ligase. Bioorg Med Chem Lett 12:679–699
Sunduru N, Gupta L, Chaturvedi V, Dwivedi R, Sinha S, Chauhan P (2010) Discovery of new 1,3,5-triazine scaffolds with potent activity against Mycobacterium tuberculosis H37Rv. Eur J Med Chem 45:3335–3345
Tenover F (2006) Mechanism of antimicrobial resistance in bacteria. Am J Med 119:S3–S10
Acknowledgments
Authors wish to thank the Department of Applied Chemistry, S. V. National Institute of Technology, Surat, for providing laboratory facilities and scholarships. The authors wish to offer their deep gratitude to Mr. Paresh Kapopara, Central and Kashiba Diagnostic Laboratory and Microbial Research Centre, Surat for carrying out biological screening. The authors are thankful to SAIF, Chandigarh, Panjab, for carrying out spectral analysis.
Conflict of interest
The authors report no declarations of interest.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Rana, A.M., Trivedi, P., Desai, K.R. et al. Novel S-triazine accommodated 5-benzylidino-4-thiazolidinones: synthesis and in vitro biological evaluations. Med Chem Res 23, 4320–4336 (2014). https://doi.org/10.1007/s00044-014-0995-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00044-014-0995-z