Abstract
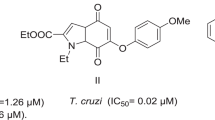
A computation model has been developed for the rational design of bioactive pharmacophore sites as anti-Mycobacterium tuberculosis and anti-Trypanosoma cruzi (TC) candidates. The 40 compounds 1–40 analyzed have been previously screened for their antitubercular and antitrypanosomal activity. The highest anti-TC activity is obtained for compounds 8 and 18 which exhibited low IC50 values (9.2 and 10.8 μM), almost equal to clinical drug, nifurtimox (7.7 μM; 100 % Inhib.). This could be attributed to the existence of two synergic (Oδ−–Nδ−) and (Oδ−–Oδ−) antitrypanosomal pharmacophore sites. In contrast to compounds 8 and 18 which contain electro-attractor groups (R1, R2 = F), analog compounds 1 and 13 with electro-donor or only hydrogen (R1, R2 = CH3, H) show best antibacterial activity (MIC = 0.977 and 1.190 μg/mL) very close to antitubercular activity of Rifampicin (MIC = 0.125 μg/mL). This could be attributed to the existence of (Oδ−–NHδ+) antibacterial pharmacophore site.




Similar content being viewed by others
References
Akkurt M, Yildirim SO, Badri R, Kerbal A, Hadda TB, Buyukgungor O (2006) 4-p-Anisyl-1,2′-diphenyl-3-p-tolyl-2′,3′-dihydrospiro [2-pyrazoline-5,3′-isoquinolin]-4′(1′H)-one. Acta Crystallogr Sect E 62(11):o5022–o5023
Akkurt M, Yıldırım SÖ, Kerbal A, Bennani B, Daoudi M, Chohan ZH, McKee V, Hadda TB (2010) Crystal structure of 3′-(4-methoxyphenyl)-2-phenyl-4′-(4-ethoxyphenyl)-1, 2-dihydro-4H, 4′ H-Spiro [isoquinoline-3, 5′-isoxazol]-4-one. J Chem Crystallogr 40(3):231–234
Al Houari G, Kerbal A, Larbi NB, Bennani B, Hadda TB, Stoeckli-Evans H (2008a) 4′-Methyl-3-(4-nitrophenyl)-4-phenyl-4,5,1′,2′,3′,4′-hexahydrospiro [isoxazole-5,2′-naphthalen]-1′-one. Acta Crystallogr Sect E 64(2):o509–o510
Al Houari G, Kerbal A, Bennani B, Baba MF, Daoudi M, Hadda TB (2008b) Drug design of new antitubercular agents: 1,3-dipolar cycloaddition reaction of para-substituted-benzadoximes and 3-para-methoxy-benzyliden-isochroman-4-ones. Arkivoc 12:42–50
Ali P, Meshram J, Sheikh J, Tiwari V, Dongre R, Hadda TB (2012) Predictions and correlations of structure activity relationship of some aminoantipyrine derivatives on the basis of theoretical and experimental ground. Med Chem Res 21(2):157–164
Anaflous A, Benchat N, Mimouni M, Abouricha S, Hadda TB, El-Bali B, Hakkou A, Hacht B (2004) Armed imidazo [1,2-a] pyrimidines (pyridines): evaluation of antibacterial activity. Lett Drug Des Discov 1:224–229
Ancizu S, Moreno E, Torres E, Burguete A, Pérez-Silanes S, Benítez D, Villar R, Solano B, Marín A, Aldana I, Cerecetto H, González M, Monge A (2009) Heterocyclic-2-carboxylic acid (3-cyano-1,4-di-noxidequinoxalin-2-yl)amide derivatives as hits for the development of neglected disease drugs. Molecules 14:2256–2272
Badri R, Kerbal A, Najib B, El-Bali B, Escudie J, Ranaivonjatovo H, Bolte M (1999) 3′-(p-Nitrophenyl)-2-phenyl-4′-(p-tolyl)spiro[isoquinoline-3(2H),5′(4′H)-isoxazolin]-4(1H) one. Acta Crystallogr 55:9900165
Bennani B, Filali Baba B, El-Fazazi A, Houari GA, Bilit N, Kerbal A, El-Bali B, Bolte M (2002) 2′,3′-Diphenyl-4′-p-tolylspiro [isothiachroman-3,5′-isoxazolidin]-4(2H)-one. Acta Crystallogr Sect E 58(3):o312–o313
Bennani B, Filali Baba B, Larbi NB, Boukir A, Kerbal A, Mimouni M, Hadda TB, Trzaskowski B, Jalbout AF (2007a) Synthesis of new spiro-[isothiochromene-3,5′-isoxazolidin]-4(1H)-ones. J Heterocycl Chem 44(3):711–716
Bennani B, Kerbal A, Daoudi M, Filali-Baba B, Houari GA, Jalbout AF, Mimouni M, Benazza M, Demailly G, Akkurt M, Yýldýrým SÖ, Hadda TB (2007b) Combined drug design of potential Mycobacterium tuberculosis and HIV-1 inhibitors: 3′,4′-di-substituted -4′H-spiro [isothiochromene-3,5′-isoxazol]-4(1H)-one. Arkivoc 16:19–40
Castro JA, de Diaz Toranzo EG (1988) Toxic effects of nifurtimox and benznidazole, two drugs used against American trypanosomiasis (Chagas’ disease). Biomed Environ Sci 1(1):19–33
Changtam C, de Koning HP, Ibrahim H, Sajid MS, Gould MK, Suksamrarn A (2010) Curcuminoid analogs with potent activity against Trypanosoma and Leishmania species. Eur J Med Chem 45:941–956
Chohan ZH, Youssoufi MH, Jarrahpour A, Hadda TB (2010) Identification of antibacterial and antifungal pharmacophore sites for potent bacteria and fungi inhibition: indolenyl sulphonamide derivatives. Eur J Med Chem 45:1189–1199
Chohan ZH, Shad HA, Toupet L, Hadda TB, Akkurt M (2011) Structure of a new bioactive agent containing combined antibacterial and antifungal pharmacophore sites: 4-{[(E)-(5-Bromo-2-hydroxyphenyl) methylidene] amino}-N-(5-methyl-1,2-oxazol-3-yl) benzenesulfonamide. J Chem Crystallogr 41(2):159–162
Ertl P, Rohde B, Selzer P (2000) Fast calculation of molecular polar surface area (PSA) as a sum of fragment-based contributions and its application to the prediction of drug transport properties. J Med Chem 43:3714–3717
Hadda TB, Badri R, Kerbal A, Bennani B, Houari GA, Filali-Baba B, Akkurt M, Demailly G, Benazza M (2008) Looking for a new antitubercular pharmacophore site: synthesis and bioactivity of spiroheterocycles 2,3′,4′-tri-subsituted-1,2-dihydro-4H, 4′H-spiro [isoquinoline-3,5′-isoxazol]-4-ones. Arkivoc 2:1–13
Hadda TB, Kerbal A, Bennani B, Houari GA, Daoudi M, Leite CL, Masand VH, Jawarkar RD, Charrouf Z (2013) Molecular drug design, synthesis and pharmacophore site identification of spiroheterocyclic compounds: trypanosoma crusi inhibiting studies. Med Chem Res 22(1):57–69
Moll C, Peris C, Moreno A, Muñoz J, Guañabens N (2008) Severe invalidating pain syndrome associated with benznidazole therapy for Chagas’ disease. Clin Rheumatol 27(2):269–270
Orhan I, Şenol FS, Khan MTH, Hadda TB, Bennani B, Kerbal A, Şener B (2009) Acetylcholinesterase inhibitory potentials of several synthetic spiro-heterocycles. International symposium on pharmaceutical sciences, Ankara, June 23–26
Parvez A, Meshram J, Tiwari V, Sheikh J, Dongre R, Youssoufi MH, Hadda TB (2010) Pharmacophores modeling in terms of prediction of theoretical physicochemical properties and verification by experimental correlations of novel coumarin derivatives produced via Betti’s protocol. Eur J Med Chem 45:4370–4378
Pinazo M-J, Muñoz J, Posada E, López-Chejade P, Gállego M, Ayala E, Del Cacho E, Soy D, Gascon J (2010) Tolerance of benznidazole in treatment of Chagas’ disease in adults. Antimicrob Agents Chemother 54(11):4896–4899
Rauf A, Liaqat S, Qureshi AM, Yaqub M, Rehman AU, Hassan MU, Chohan ZH, Nasim Faiz UH, Hadda TB (2010) Synthesis, characterization, and urease inhibition of 5-substituted-8-methyl-2H-pyrido [1,2-a] pyrimidine-2, 4 (3H)-diones. Med Chem Res 21(1):60–74
Sheikh J, Parvez A, Juneja H, Ingle V, Chohan Z, Youssoufi M, Hadda TB (2011) Synthesis, biopharmaceutical characterization, antimicrobial and antioxidant activities of 1-(4′-O-β-D-glucopyranosyloxy-2′-hydroxyphenyl)-3-aryl-propane-1,3-diones. Eur J Med Chem 46:1390–1399
Viotti R, Vigliano C, Lococo B, Alvarez MG, Petti M, Bertocchi G, Armenti A (2009) Side effects of benznidazole as treatment in chronic Chagas disease: fears and realities. Expert Rev Anti Infect Ther 7(2):157–163
Acknowledgments
Prof. T. Ben Hadda and Dr. J. Sheikh would like to thank the ACTELION; the Biopharmaceutical Company of Swiss, for molecular properties calculations. The authors extend their appreciation to the Deanship of Scientific Research at King Saud University for funding the work through the research group project Number RGP-VPP-007.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hadda, T.B., Bendaha, H., Sheikh, J. et al. Computational POM evaluation of experimental in vitro Trypanosoma cruzi and Mycobacterium tuberculosis inhibition of heterocyclic-2-carboxylic acid (3-cyano-1,4-di-noxidequinoxalin-2-yl)amide derivatives. Med Chem Res 23, 1956–1965 (2014). https://doi.org/10.1007/s00044-013-0781-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00044-013-0781-3