Abstract
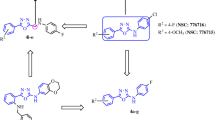
Two novel series of heterocyclic compounds have been synthesized. In first series, isatin was allowed to react with substituted aromatic/cyclic carbonyl compounds to get desired mannich bases (2a–e). In second series, 4,5-disubstituted oxazoles (6a–p) were synthesized. Eight compounds (2c, 6a, 6e, 6f, 6i, 6j, 6m, and 6n) were screened for anticancer activity in 60 cell lines. Compound 2c, 1-[(4,7,7-trimethyl-3-oxobicyclo[2.2.1]heptan-2-yl)methyl]indoline-2,3-dione, showed maximum activity and thus, selected for further evaluation at five dose level screening. Furthermore, molecular docking studies of compounds 2c into the colchicine-binding site of tubulin, revealed possible mode of inhibition by the compound.






Similar content being viewed by others
References
Alley MC, Scudiero DA, Monks AP, Hursey ML, Czerwinski MJ, Fine DL, Abbott BJ, Mayo JG, Shoemaker RH, Boyd MR (1988) Feasibility of drug screening with panels of human tumor cell lines using a microculture tetrazolium assay. Cancer Res 48:589–601
Bai R, Covell DG, Pei XF, Ewell JB, Nguyen NY, Brossi A, Hamel E (2000) Mapping the binding site of colchicinoids on beta-tubulin. 2-Chloroacetyl-2-demethylthiocolchicine covalently reacts predominantly with cysteine 239 and secondarily with cysteine 354. J Biol Chem 275:40443–40452
Bailly C, Bal C, Barbier P, Comber S, Finet JP, Hildebrand MP, Peyrot V, Wattez N (2003) Synthesis and biological evaluation 4-arylcoumarin analogues of combretastatins. J Med Chem 46:5437–5444
Bhat KP, Pezzuto JM (2001) Resveratrol exhibits cytostatic and antiestrogenic properties with human endometrial adenocarcinoma (Ishikawa) cells. Cancer Res 61:6137–6144
Boyd MR, Paull KD (1995) Some practical considerations and applications of the national cancer institute in vitro anticancer drug discovery screen. Drug Dev Res 34:91–109
Burns RG (1992) Analysis of the colchicine-binding site of beta-tubulin. FEBS Lett 297:205–208
Cai SX (2007) Small molecule vascular disrupting agents: potential new drugs for cancer treatment. Recent Pat Anticancer Drug Discov 2:79–101
Ciolino HP, Yeh GC (2001) The effects of resveratrol on CYP1A1 expression and aryl hydrocarbon receptor function in vitro. Adv Exp Med Biol 492:183–193
De Martino G, La Regina G, Coluccia A, Edler MC, Barbera MC, Brancale A, Wilcox E, Hamel E, Artico M, Silvestri R (2004) Arylthioindoles, potent inhibitors of tubulin polymerization. J Med Chem 47:6120–6123
De Martino G, Edler MC, La Regina G, Coluccia A, Barbera MC, Barrow D, Nicholson RI, Chiosis G, Brancale A, Hamel E (2006) New arylthioindoles: potent inhibitors of tubulin polymerization. 2. Structure-activity relationships and molecular modeling studies. J Med Chem 49:947–954
DeVita VT, Hellman S, Rosenberg SA (1989) Cancer: principles and practice of oncology updates. Lippincott, Philadelphia
Do Yoon K, Kim KH, Kim ND, Lee KY, Han CK, Yoon JH, Moon SK, Lee SS, Seong BL (2006) Design and biological evaluation of novel tubulin inhibitors as antimitotic agents using pharmacophore binding model with tubulin. J Med Chem 49:5664–5670
Dupeyre G, Chabot GG, Thoret S, Cachet X, Seguin J, Guenard D, Tillequin F, Scherman D, Koch M, Michel S (2006) Synthesis and biological evaluation of (3,4,5-trimethoxyphenyl)indol-3-ylmethane derivatives as potential antivascular agents. Bioorg Med Chem 14:4410–4426
Flynn BL, Hamel E, Jung MK (2002) One-pot synthesis of benzo[b]furan and indole inhibitors of tubulin polymerization. J Med Chem 45:2670–2673
Friesner RA, Banks JL, Murphy RB, Halgren TA, Klicic JJ, Mainz DT, Repasky MP, Knoll EH, Shelley M, Perry JK (2004) Glide: a new approach for rapid, accurate docking and scoring. 1. Method and assessment of docking accuracy. J Med Chem 47:1739–1749
Gaukroger K, Hadfield JA, Hepworth LA, Lawrence NJ, McGown AT (2001) Novel synthesis of cis and trans isomers of combretastatin A-4. J Org Chem 66:8135–8138
Glide, version 5.5 (2009) Schrödinger, LLC, New York
Halgren TA, Murphy RB, Friesner RA, Beard HS, Frye LL, Pollard WT, Banks JL (2004) Glide: a new approach for rapid, accurate docking and scoring. 2. Enrichment factors in database screening. J Med Chem 47:1750–1759
Hinnen P, Eskens FALM (2007) Vascular disrupting agents in clinical development. Br J Cancer 96:1159–1165
Horsman MR, Siemann DW (2006) Pathophysiologic effects of vascular-targeting agents and the implications for combination with conventional therapies. Cancer Res 66:11520–11539
Jang M, Cai L, Udeani GO, Slowing KV, Thomas CF, Beecher CW, Fong HHS, Farnsworth NR, Kinghorn AD, Mehta RG (1997) Cancer chemopreventive activity of resveratrol, a natural product derived from grapes. Science 275:218–220
Kaffy J, Pontikis R, Carrez D, Croisy A, Monneret C, Florent JC (2006) Isoxazole-type derivatives related to combretastatin A-4, synthesis and biological evaluation. Bioorg Med Chem 14:4067–4077
Kang JH, Park YH, Choi SW, Yang EK, Lee WJ (2003) Resveratrol derivatives potently induce apoptosis in human promyelocytic leukemia cells. Exp Mol Med 35:467–474
La Regina G, Edler MC, Brancale A, Kandil S, Coluccia A, Piscitelli F, Hamel E, De Martino G, Matesanz R, Díaz JF (2007) Arylthioindole inhibitors of tubulin polymerization. 3. Biological evaluation, structure-activity relationships and molecular modeling studies. J Med Chem 50:2865–2874
Latruffe N, Delmas D, Jannin B, Cherkaoui Malki M, Passilly-Degrace P, Berlot JP (2002) Molecular analysis on the chemopreventive properties of resveratrol, a plant polyphenol microcomponent. Int J Mol Med 10:755–760
Lin CM, Singh SB, Chu PS, Dempcy RO, Schmidt JM, Pettit GR, Hamel E (1988) Interactions of tubulin with potent natural and synthetic analogs of the antimitotic agent combretastatin: a structure activity study. Mol Pharmacol 34:200–208
Lin CM, Ho HH, Pettit GR, Hamel E (1989) Antimitotic natural products combretastatin A-4 and combretastatin A-2: studies on the mechanism of their inhibition of the binding of colchicine to tubulin. Biochemistry 28:6984–6991
Liou JP, Chang JY, Chang CW, Chang CY, Mahindroo N, Kuo FM, Hsieh HP (2004) Synthesis and structure-activity relationship of 3-aminobenzophenones as antimitotic. J Med Chem 47:2897–2905
Lippert JW III (2007) Vascular disrupting agents. Bioorg Med Chem 15:605–615
Murias M, Jager W, Handler N, Erker T, Horvath Z, Szekeres T, Nohl H, Gille L (2005) Antioxidant, prooxidant and cytotoxic activity of hydroxylated resveratrol analogues: structure-activity relationship. Biochem Pharmacol 69:903–912
Ohsumi K, Hatanaka T, Fujita K, Nakagawa R, Fukuda Y, Nihei Y, Suga Y, Morinaga Y, Akiyama Y, Tsuji T (1998) Syntheses and antitumor activity of cis-restricted combretastatins: 5-membered heterocyclic analogues. Bioorg Med Chem Lett 8:3153–3158
Pandit B, Sun Y, Chen P, Sackett DL, Hu Z, Rich W, Li C, Lewis A, Schaefer K, Li PK (2006) Structure-activity-relationship studies of conformationally restricted analogs of combretastatin A-4 derived from SU5416. Bioorg Med Chem 14:6492–6501
Patterson DM, Rustin GJS (2007) Vascular damaging agents. Clin Oncol 19:443–456
Peifer C, Stoiber T, Unger E, Totzke F, Schachtele C, Marme D, Brenk R, Klebe G, Schollmeyer D, Dannhardt G (2006) Design, synthesis, and biological evaluation of 3,4-diarylmaleimides as angiogenesis inhibitors. J Med Chem 49:1271–1281
Pettit GR, Singh SB, Hamel E, Lin CM, Alberts DS, Garcia- Kendall D (1989) Isolation and structure of the strong cell growth and tubulin inhibitor combretastatin A-4. Cell Mol Life Sci 45:209–211
Pettit GR, Singh SB, Boyd MR, Hamel E, Pettit RK, Schmidt JM, Hogan F (1995) Antineoplastic agents-291. Isolation and synthesis of combretastatin A-4, A-5 and A-6. J Med Chem 38:1666–1672
Pettit GR, Rhodes MR, Herald DL, Chaplin DJ, Stratford MRL, Hamel E, Pettit RK, Chapuis JC, Oliva D (1998) Antineoplastic agent 393. Synthesis of the trans isomer of combretastatin A-4 prodrug. Anticancer Drug Des 13:981–993
Pettit GR, Rhodes MR, Herald DL, Hamel E, Schmidt JM, Pettit RK (2005) Antineoplastic agents. 445. Synthesis and evaluation of structural modification of (Z)- and (E)-combretastatin A-4. J Med Chem 48:4087–4099
Ravelli RBG, Gigant B, Curmi PA, Jourdain I, Lachkar S, Sobel A (2004) Insight into tubulin regulation from a complex with colchicine and a stathmin-like domain. Nature 428:198–202
Rostom SAF (2006) Synthesis and in vitro antitumor evaluation of some indeno[1,2-c]pyrazol(in)es substituted with sulfonamide, sulfonylurea(-thiourea) pharmacophores, and some derived thiazole ring systems. Bioorg Med Chem 14:6475–6485
Shearwin KE, Timasheff SN (1994) Effect of colchicine analogues on the dissociation of alpha beta tubulin into subunits: the locus of colchicine binding. Biochemistry 33:894–901
Simoni D, Grisolia G, Giannini G, Roberti M, Rondanin R, Piccagli L, Baruchello R, Rossi M, Romagnali R, Invidiata FP, Grimaudo S, Jung MK, Hamel E, Gebbia N, Crosta L, Abbadessa V, Cristina AD, Dusonchet L, Meli M, Tolomeo M (2005) Heterocyclic and phenyl double-bond-locked combretastatin analogues possessing potent apoptosis-inducing activity in HL-60 and in MDR cell lines. J Med Chem 48:723–736
Simoni D, Romagnoli R, Baruchello R, Rondanin R, Rizzi M, Pavani MG, Alloatti D, Giannini G, Marcellini M, Riccioni T, Castorina M, Guglielmi MB, Bucci F, Carminati P, Pisano C (2006) Novel combretastatin analogues endowed with antitumor activity. J Med Chem 49:3143–3152
Solomon VR, Hu C, Lee H (2009) Hybrid pharmacophore design and synthesis of isatin–benzothiazole analogs for their anti-breast cancer activity. Bioorg Med Chem 17:7585–7592
SYBYL 7.1 (2005) Tripos Inc., St. Louis
Tahir SK, Nukkala MA, Zielinski Mozny NA, Credo RB, Warner RB, Li Q, Woods KW, Claiborne A, Gwaltney SLII, Frost DJ, Sham HL, Rosenberg SH, Ng SC (2003) Biological activity of A-289099: on orally active tubulin-binding indolyloxazoline derivative. Mol Cancer Ther 2:227–233
Tron GC, Pirali T, Sorba G, Pagliai F, Busacca S, Genazzani AA (2006) Medicinal chemistry of combretastatin A-4: present and future directions. J Med Chem 49:3033–3044
Vine KL, Matesic L, Locke JM, Ranson M, Skropeta D (2009) Cytotoxic and anticancer activities of isatin and its derivatives: a comprehensive review from 2000–2008. Anticancer Agents in Med Chem 9:397–414
Wang L, Woods KW, Li O, Barr KJ, McCroskey RW, Hennick SM, Gherke L, Credo RB, Hui YH, Marsh K, Warner R, Lee JY, Zieliniki–Mozng N, Frost D, Rosenberg SH, Shan HL (2002) Potent, orally active heterocycle-based combretastatin A-4 analogues: synthesis, structure-activity relationship, pharmacokinetics, and in vivo antitumor activity evaluation. J Med Chem 45:1697–1711
West CML, Price P (2004) Combretastatin A4 phosphate. Anticancer Drugs 15:179–187
Wilson L, Bamburg JR, Mizel SB, Grisham LM, Creswell KM (1974) Interaction of drugs with microtubule proteins. Fed Proc 33:158–166
Woods JA, Hadfield JA, Pettit GR, Fox BW, McGown AT (1995) The interaction with tubulin of a series of stilbenes based on combretastatin A-4. Br J Cancer 71:705–711
Xia Y, Yang ZY, Xia P, Bastow KF, Tachibana Y, Kuo SC, Hamel E, Hackl T, Lee KH (1998) Antitumor agents. 181. Synthesis and biological evaluation of 6,7,2′,3′,4′-substituted-1,2,3,4-tetrahydro-2-phenyl-4-quinolones as a new class of antimitotic antitumor agents. J Med Chem 41:1155–1162
Acknowledgments
The author’s are thankful to the staff members of National Cancer Institute (NCI), USA, for in vitro anticancer screening of the newly synthesized compounds.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chaudhary, A., Sharma, P.P., Bhardwaj, G. et al. Synthesis, biological evaluation, and molecular modeling studies of novel heterocyclic compounds as anti-proliferative agents. Med Chem Res 22, 5654–5669 (2013). https://doi.org/10.1007/s00044-013-0556-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00044-013-0556-x