Abstract
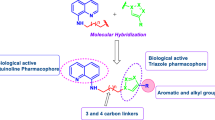
A series of novel hybrid 4-aminoquinoline-1,3,5-triazine derivatives were developed and subsequently tested against representative Gram-positive and Gram-negative microorganisms for determination of their antibacterial activity. Screening results indicate that, title molecule exhibit moderate to potent activity in comparison to standard. These hybrid derivatives were synthesized through a facile synthetic routes and structure of reaction intermediates as well as target molecules were recognised with the aid of various spectroscopic techniques viz., FTIR, NMR, mass and elemental analysis.



Similar content being viewed by others
References
Alanis AJ (2005) Resistance to antibiotics: are we in the post-antibiotic era. Arch Med Res 36:697–705
Bhat HR, Singh UP, Yadav PS, Kumar V, Das A, Chetia D, Prakash A, Mahanta J (2011) Synthesis, characterization and antimalarial activity of hybrid 4-aminoquinoline-1,3,5-triazine derivatives. Arabian J Chem. doi:10.1016/j.arabjc.2011.07.001
Bhat HR, Ghosh SK, Prakash A, Gogoi K, Singh UP (2012a) In vitro antimalarial activity and molecular docking analysis of 4-aminoquinoline-clubbed 1,3,5-triazine derivatives. Lett Appl Microbiol 54:483–486
Bhat HR, Gupta SK, Singh UP (2012b) Discovery of potent, novel antibacterial hybrid conjugates from 4-aminoquinoline and 1,3,5-triazine: design, synthesis and antibacterial evaluation. RSC Adv 2:12690–12695
Bhat HR, Singh UP, Gahtori P, Ghosh SK, Gogoi K, Prakash A, Singh RK (2013) Antimalarial activity and docking studies of novel bi-functional hybrids derived from 4-aminoquinoline and 1,3,5-triazine against wild and mutant malaria parasites as pf-DHFR inhibitor. RSC Adv. doi:10.1039/C2RA21915H
Corbett TH, Leopold WR, Dykes DJ, Roberts BJ, Griswold DP Jr, Schabel FM Jr (1982) Toxicity and anticancer activity of a new triazine antifolate (NSC 127755). Cancer Res 42:1707–1715
Davies J, Davies D (2010) Origins and evolution of antibiotic resistance. Microbiol Mol Biol 74:417–733
Dubey V, Pathak M, Bhat HR, Singh UP (2012) Design, facile synthesis and antibacterial activity of hybrid 1,3,4-thiadiazole-1,3,5-triazine derivatives tethered via S-bridge. Chem Biol Drug Des 80:598–604
Gahtori P, Ghosh SK, Singh B, Singh UP, Bhat HR, Uppal A (2012) Synthesis, SAR and antibacterial activity of hybrid chloro, dichloro-phenylthiazolyl-s-triazines. Saudi Pharm J 20:35–43
Ghosh SK, Saha A, Hazarika B, Singh UP, Bhat HR, Gahtori P (2012) Design, facile synthesis, antibacterial activity and structure-activity relationship of novel di- and tri-substituted 1,3,5-triazines. Lett Drug Des Dis 9:329–335
Junejo JA, Ghosh SK, Shaikh M, Gahtori P, Singh UP (2011) Facile synthesis, antibacterial activity and molecular properties prediction of Some new 1,3-dihydroimidazol-2-thione derivatives. Lett Drug Des Dis 8:763–768
Kumar S, Bhat HR, Kumawat MK, Singh UP (2013) Design and one-pot synthesis of hybrid thiazolidin-4-one-1,3,5-triazines as potent antibacterial agent against human disease causing pathogens. New J Chem. doi:10.1039/C2NJ41028A
Lozano V, Aguado L, Hoorelbeke B, Renders M, Camarasa MJ, Schols D, Balzarini J, San-Félix A, Pérez-Pérez MJ (2011) Targeting HIV entry through interaction with envelope glycoprotein 120 (gp120): synthesis and antiviral evaluation of 1,3,5-triazines with aromatic amino acids. J Med Chem 54:5335–5348
Martinez JL, Baquero F (2000) Mutation frequencies and antibiotic resistance. Antimicrob Agents Chemother 44:1771–1777
National Committee for Clinical Laboratory Standards (1982) Standard methods for dilution antimicrobial susceptibility test for bacteria which grow aerobically. NCCLS, Villanova, p 242
Shapiro RS, Robbins N, Cowen LE (2011) Regulatory circuitry governing fungal development, drug resistance, and disease. Microbiol Mol Biol Rev 75:213–267
Singh UP, Singh RK, Bhat HR, Subhaschandra YP, Kumar V, Kumawat MK, Gahtori P (2011) Synthesis and antibacterial evaluation of a series of novel trisubstituted-s-triazines derivatives. Med Chem Res 20:1603–1610
Singh UP, Bhat HR, Gahtori P (2012a) Antifungal activity, SAR and physicochemical correlation of some thiazole-1,3,5-triazine derivatives. J Mycol Med 22:134–141
Singh UP, Pathak M, Dubey V, Bhat HR, Gahtori P, Singh RK (2012b) Design, synthesis, antibacterial activity and molecular docking studies on novel hybrid 1,3-thiazine-1,3,5-triazine derivatives as potential bacterial translation inhibitor. Chem Biol Drug Des 80:572–583
Singh UP, Bhat HR, Gahtori P, Singh RK (2013) Hybrid thiazole-1,3,5-triazines target cytosolic Leucyl-tRNA synthetase for antifungal action revealed by molecular docking studies. In Silico Pharmacology, In press
Singh UP, Bhat HR, Singh RK (2013b) Ceric ammonium nitrate catalysed expeditious one-pot synthesis of 1,3-thiazine as IspE kinase inhibitor of gram-negative bacteria using polyethylene glycol (PEG-400) as an efficient recyclable reaction medium. C. R. Chime. doi:10.1016/j.crci.2012.11.019
Acknowledgments
Authors are gratified to SAIF, Central Drug Research Institute, Lucknow, India for providing spectral data of compounds synthesized herein and SHIATS for providing basic facilities to carry out the project.
Conflict of interest
Authors declare no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Bhat, H.R., Pandey, P.K., Ghosh, S.K. et al. Development of 4-aminoquinoline-1,3,5-triazine conjugates as potent antibacterial agent through facile synthetic route. Med Chem Res 22, 5056–5065 (2013). https://doi.org/10.1007/s00044-013-0521-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00044-013-0521-8