Abstract
The two-stages studies of structure–activity relationship for model ligands of 5HT1A, 5HT2A, and D2 receptors were performed. On the first stage, the pharmacophores of two potential ligands of known in vitro binding to 5HT1A, 5HT2A, D2 receptors and model pharmacophore of strongly interacting D2 receptor ligands were found and their parameters were related to affinity data. The analyzed parameters were hydrophobic, hydrophilic, aromatic, donor and acceptor of proton centers. The geometry of spatial distribution of these properties was also investigated in comparative analysis. The studied, model compounds were two 3β-acylamine derivatives of tropane. The second stage includes docking of studied compounds to D2 receptor model and the comparison of its quality with in vivo binding data. The obtained results are consistent with in vitro binding data and applied procedure accurate estimates the affinity of potential ligands to D2 receptors.
Similar content being viewed by others
Introduction
In commonly accepted opinion every searching for new, more effective drugs should be rationalized i.e., determined by the low cost and non time-consuming procedures. These procedures are especially useful on the preliminary stage of searching for new chemical structures of potential biological activity (Jorgensen, 2004; Leeson and Springthorpe, 2007; Ou-Yang et al., 2012). In general, on this purpose there are employed various correlation QSAR methods (Dudek et al., 2006; Yang and Huang, 2006; Shailesh et al., 2012). However, in particular cases it is more convenient to develop the procedure of selection of the appropriate structures based on more direct and easier interpretatively criteria. It seems that just such a case is a search for effective ligands of 5HT1A, 5HT2A, and D2 receptors since many structural data on their agonist and antagonist as well as the models of these receptors are well-known (Klabunde and Hessler, 2002; Bissantz et al., 2003; Teeter et al., 1994; Chambers and Nichols, 2002; Homan et al., 1999). In addition, wide availability of various bases containing a lot of structural data on very active ligands allows to generate pretty accurate pharmacophore patterns (Nelson, 1991; Bojarski, 2006). Thanks to these all literature data it is possible to estimate the affinity of potential ligand for receptor of interest. The chemical structure of pharmacophore of being selected potential ligand and its affinity to the receptor seem to be sufficiently unambiguous discriminators, on a preliminary stage, in the search for new effective antipsychotics. To verify this hypothesis, the two-step procedure was developed and tested. The first step includes determination of pharmacophores for two tested compounds of well-known affinity (previously in vitro determined) to the same receptors as well as pharmacophore pertinent to well-known D2 receptor agonists or antagonists and finally comparison of their properties to in vitro binding data. The pharmacophore model of D2 receptor ligands was found on the basis of 15 compounds of high affinity to D2 receptor reported in literature (Słowiński et al., 2011). These two tested compounds were 3β-acylamine derivatives of tropane: N-(8-Furan-2-ylmethyl-8-azabicyclo[3.2.1]oct-3β-yl)-2-methoxybenzamide (compounds I) and N-(8-Furan-2-ylmethyl-8-azabicyclo[3.2.1]oct-3β-yl)-2.3-dimethoxybenzamide (compound II) (Fig. 1). Their synthesis have been developed and described in the previously published paper on tropane derivatives (Słowiński et al., 2011).
The pharmacophores of compounds I and II were found on the basis of their structures determined by X-ray diffraction method. The CCDC (Cambridge Crystallographic Data Centre) numbers of compounds I and II are: 905689 and 905690, respectively (Figs. 2, 3).
The molecular structure of compound I shows an intramolecular hydrogen bond between the O atom of the methoxy group and the NH of the amide function leads to a six-membered ring. The dihedral angle between the least-squares planes of the phenyl and this virtual ring is only 2.50(7)°. The piperidine moiety adopts a chair conformation. The substituent at N8 is in an equatorial position. The best plane of the furan ring and the C1/C2/C4/C5 plane make an angle 69.42(9)° and the dihedral angle between the planes of the furan and benzene rings is 72.50(8)°.
The compound II molecule adopts a folded conformation with an angle between the furan and benzene rings of 63.29(8)° and between the best plane of the furan ring and the C1/C2/C4/C5 plane of 87.56(9)°. This conformation is stabilized by an intramolecular N15–H15A···O25 and C26–H26C···O27 hydrogen bonds. As a result of N15–H15A···O25 interaction a six-membered ring is formed and make an angle 9.2(1)° with the phenyl ring. The piperidine moiety assumes a chair conformation and the substituent at N8 is in an equatorial position. Conformations of both methoxy groups are different. The disposition of these groups with respect to the phenyl ring can be described by the torsion angles C18–C19–O25–C26 of −107.8(2)° and C21–C20–O27–C28 of 11.1(3)°. In consequence, the methyl carbon atom C26 is found to be 1.107(4) Å out of the phenyl plane, and C28 atom is almost coplanar with this ring.
The pharmacophore structure is a reflection template of the geometrical distribution of property centers localized in molecule and determines to large extent its biological activity. It means that even subtle differences in the geometry of structurally similar molecules can significantly impact on their affinity to receptor binding site.
The comparative analysis of the studied pharmacophores was intended to find the specific properties and geometrical parameters which are crucial for the strength of binding of potential ligands to the receptors of interest.
The second step of the applied procedure devoted to the selection of the potential agonists or antagonists of the studied receptors relies on docking of the reference compounds I and II to the models of the D2 receptor (Sakhteman et al., 2011). From analysis of in vitro results (Table 1) follows that the both studied compounds (I, II) are very poorly being bounded to 5-HT1A and 5-HT2A receptors. Indeed, the model docking of compounds I and II to these receptors also showed that such binding cannot take place. The both molecules of compounds I and II were placed outside the receptor binding pockets. Thus, only docking of compounds I and II to D2 receptor is detailed analyzed. The most discriminative parameters which distinctly classify the quality of docking are number and strength (equivalently length and geometry) of the hydrogen bonds formed between ligand and specific amino acids not only inside the receptor binding pocket but also, although to a less degree, intermolecular interactions of other types e.g., hydrophobic and edge-to-face.
The used 3D homology model of D2 receptor has been revealed by comparative modeling using the crystal structure of the human β2-adrenergic receptor and the bovine rhodopsin as the templates (Sakhteman et al., 2011; Strzelczyk et al., 2004; Wang et al., 2010). The quite recently reported X-ray structure of the human β2-adrenergic receptor opens new possibilities for modeling of the correct structures of the dopamine ones. Currently, the human β2-adrenergic receptor is considered to be more homologous to the dopamine receptors than bovine rhodopsin (Cherezov et al., 2007). All modeling of the pharmacophores as well as docking of the compounds I and II to the D2 receptor model were done by Discovery Studio software (Accelrys Software Inc., Discovery Studio Modeling Environment, 2005).
Materials and methods
X-ray diffraction measurements
Crystals of compounds I and II suitable for X-ray analysis were grown by slow evaporation from acetate/diisopropyl ether (compound I) and hexane/ethanol (compound II) solutions. The data were collected on an Oxford Diffraction KM4CCD diffractometer at 293 K, using graphite-monochromated Mo Kα radiation. The unit cell parameters were determined by least-squares treatment of setting angles of highest-intensity reflections chosen from the whole experiment. Intensity data were corrected for the Lorentz and polarization effects. The structure was solved by direct methods using the SHELXS97 program (Sheldric, 1990) and refined by the full-matrix least-squares method with the SHELXL97 program (Sheldric, 1997). The function Σw(|F o|2 − |F c|2)2 was minimized with w −1 = [σ2(F o)2 + (0.0688P)2], where P = (F 2o + 2F 2c )/3. An empirical extinction correction was also applied according to the formula F c′ = kF c[1 + (0.001χF 2c λ3/sin2θ)]−1/4 (Sheldric, 1997) and the extinction coefficient χ was equal to 0.014(2). All non-hydrogen atoms were refined anisotropically. The coordinates of the hydrogen atoms were calculated in idealized positions and refined as a riding model with their thermal parameters calculated as 1.2 (1.5 for methyl group) times Ueq of the respective carrier carbon atom.
Results and discussion
The in vitro binding data for compounds I, II as ligands of 5HT1A, 5HT2A, and D2 receptors are given in Table 1 (Słowiński et al., 2011).
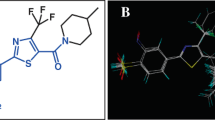
These experimental binding data unambiguously points at very low affinity of compound I to 5HT1A and 5HT2A receptors and somewhat better to D2 one, yet, compound II displayed very weak binding activity to 5HT1A, moderate to 5HT2A and very high to D2 receptors. The differences between parameters (geometrical and property types) of the reference pharmacophores and the pharmacophores pertinent to compounds I and II are expected to reflect the differences in affinity of tested compounds to the receptors of interest. The found structures of pharmacophores described by their specific properties are given on—Figs. 4, 5, and 6. The particular colors denote the following properties: red—positive ionization (nitrogen atom), green—hydrogen bond acceptor, magenta—hydrogen bond donor, pale blue—hydrophobic, aromatic, ultramarine—hydrophobic, aliphatic, orange—aromatic ring (Table 2).
The geometry of a spatial distribution of pharmacophore properties in obtained models is an exact reflection of the X-ray diffraction structure of compounds I and II (Table 3). It is worthy to note that in spite of the high similarity of chemical structures of these compounds, that their conformations significantly differ each from other. Consequently, these differences distinctly appear in pharmacophore models. Obviously, it should be taken into account some flexibility of the spatial pharmacophore geometry and possibility of its change during docking of studied compounds to particular receptors. However, such changes are often possible only to small degree or impossible at all on account of the high energetic rotation barriers. In this context, the presence of two separate aliphatic—hydrophobic centers in pharmacophore of compound II takes on a special importance for explanation of very high affinity of this compound, in contrast to compound I, for D2 receptor. It is likely that just second methoxy group in compound II molecule underlies its high binding to D2 receptor while the same group do not affect the affinity of compound II to 5-HT1A and 5-HT2A receptors. The comparative analysis of the D2 receptor ligand pharmacophore (Fig. 6) and pharmacophores of compounds I and II also leads to the same conclusion (Figs. 4 and 5). The pharmacophore of D2 ligand quite well matches the pharmacophore of compound II but does not the pharmacophore of compound I (c.f. Fig. 7). In addition, specificity of the structural relation between these pharmacophores results from the identical spatial localization of the aliphatic property of D2 ligand pharmacophore and its analog present in pharmacophore of compound II but absent in pharmacophore of compound I (Table 2).
Docking of both tested compounds to D2 receptor model turned out to be non discriminative investigation not giving criteria for explanation of difference in ability to the binding of compounds I and II with D2 receptor. Both compounds docked to D2 receptor interact with its amino acids via the same hydrogen bonds. In case of compound I the hydrogen bonds are: ligand—thyrosine 379 (length 2.198 Å), ligand—alanine 185 (length 2.315 Å), and compound II ligand—thyrosine 379 (length 2.310 Å), ligand—alanine 185 (length 2.139 Å). In addition, both compounds interact similarly with D2 receptor with hydrophobic forces (Fig. 8).
The obtained docking results are not unexpected since, purposely, the structurally similar compounds were investigated to point out that even very subtle differences in the chemical structure of compounds, to which docking procedure is “insensitive”, may impact crucially on their therapeutic activity. Thus, it should be stated that two stages “pharmacophore” and “docking” investigations are necessary to estimate properly an affinity of newly designed receptor ligands. On the whole, these studies were intended to prove that postulated two-stages procedure can be applied to verification of the properties of even very similar structurally potential and being designed antipsychotics.
References
Accelrys Software Inc. (2005-09) Discovery studio modeling environment, version 2.5.5.9350. Accelrys Software Inc, San Diego
Bissantz C, Bernard P, Hibert M, Rognan D (2003) Protein-based virtual screening of chemical databases. II. Are homology models of G-protein coupled receptors suitable targets? Proteins 50(1):5–25
Bojarski AJ (2006) Pharmacophore models for metabotropic 5-HT receptor ligands. Curr Top Med Chem 6(18):2005–2026
Chambers JJ, Nichols DE (2002) A homology-based model o the human 5-HT 2A receptor derived from an in silico activated G-protein coupled receptor. J Comput Aided Mol Des 16(7):511–520
Cherezov V, Rosenbaum DM, Hanson MA, Rasmussen SG, Thian FS, Kobilka TS, Choi HJ, Kuhn P, Weis WI, Kobilka BK, Stevens RC (2007) High-resolution crystal structure of an engineered human beta-2-adrenergic G protein-coupled receptor. Science 318:1258–1265
Dudek AZ, Arodz T, Galvez J (2006) Computational methods in developing quantitative structure–activity relationship (QSAR); a review. Comb Chem High Throughput Screen 9:213–228
Homan EJ, Wikström HV, Grol CJ (1999) Molecular modeling of the dopamine D2 and serotonin 5-HT(1A) receptor binding modes of the enantiomers of 5-OMe-BPAT. Bioorg Med Chem 7(9):1805–1820
Jorgensen WL (2004) The many roles of computation in drug discovery. Science 303(5665):1813–1818
Klabunde T, Hessler G (2002) Drug design strategies for targeting G-protein-coupled receptors. ChemBioChem 3(10):928–944
Leeson PD, Springthorpe B (2007) The influence of drug-like concepts on decision-making in medicinal chemistry. Nat Rev Drug Discov 6(11):881–890
Nelson DL (1991) Structure–activity relationships at 5-HT(1A) receptors: binding profiles and intrinsic activity. Pharmacol Biochem Behav 40(4):1041–1051
Ou-Yang S-S, Lu J-Y, Kong X-Q, Liang Z-J, Luo C, Jiang H (2012) Computational drug discovery. Acta Pharm Sin 33(9):1131–1140
Sakhteman A, Lahtela-Kakkonen M, Poso A (2011) Studying the catechol binding cavity in comparative models of human dopamine D2 receptor. J Mol Graph Model 29:685–692
Shailesh VJ, Kamlendra SB, Sanjaykumar BB (2012) QSAR and flexible docking studies of some aldose reductase inhibitors obtained from natural origin. Med Chem Res 21(8):1665–1676
Sheldric GM (1990) Phase annealing in SHELX-90; direct methods for larger structures. Acta Crystallogr A 46:467–473
Sheldric GM (1997) SHELXL97, program for the refinement of crystal structures. University of Göttingen, Göttingen
Słowiński T, Stefanowicz J, Dawidowski M, Kleps J, Czuczwar S, Andres-Mach M, Łuszczki JJ, Nowak G, Stachowicz K, Szewczyk B, Sławińska A, Mazurek AP, Mazurek A, Pluciński F, Wolska I, Herold F (2011) Synthesis and biological investgation of potential atypical antipsychotics with tropane core. Part 1. Eur J Med Chem 46:4474–4488
Strzelczyk AA, Jarończyk M, Chilmończyk Z, Mazurek AP, Chojnacka-Wójcik E, Sylte I (2004) Intrinsic activity and comparative molecular dynamics of buspirone analogues at the 5-HT1A receptors. Biochem Pharmacol 6:2219–2230
Teeter MM, Froimowitz M, Stec B, DuRand CJ (1994) Homology modeling of the dopamine D2 receptor and its testing by docking of agonists and tricyclic antagonists. J Med Chem 37(18):2874–2888
Wang Q, Mach RH, Luedtke RR, Reichert DE (2010) Subtype selectivity of dopamine receptor ligands; insights from structure and ligand-based methods. J Chem Inf Model 50:1970–1985
Yang GF, Huang X (2006) Development of quantitative structure–activity relationships and its application in rational drug design. Curr Pharm Des 12:4601–4611
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
About this article
Cite this article
Słowiński, T., Stefanowicz, J., Wróbel, M.Z. et al. Model structure–activity relationship studies of potential tropane 5HT1A, 5HT2A, and D2 receptor ligands. Med Chem Res 22, 3148–3153 (2013). https://doi.org/10.1007/s00044-012-0305-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00044-012-0305-6